Difference between revisions of "Sodium sulfate, anhydrous"
(username removed) |
(username removed) |
||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | White, hygroscopic crystals or powder. Anhydrous sodium sulfate occurs in nature as the mineral thenardite. Thenardite occurs most often as an evaporation product near salt lakes and playas, and is mined in arid regions of northern Africa, Siberia, Canada, and the western United States. Anhydrous sodium sulfate is used in the manufacture of [http://cameo.mfa.org/materials/fullrecord.asp?name=kraft | + | White, hygroscopic crystals or powder. Anhydrous sodium sulfate occurs in nature as the mineral thenardite. Thenardite occurs most often as an evaporation product near salt lakes and playas, and is mined in arid regions of northern Africa, Siberia, Canada, and the western United States. Anhydrous sodium sulfate is used in the manufacture of [http://cameo.mfa.org/materials/fullrecord.asp?name=kraft%20paper kraft paper], [http://cameo.mfa.org/materials/fullrecord.asp?name=paperboard paperboard], [http://cameo.mfa.org/materials/fullrecord.asp?name=glass glass], [http://cameo.mfa.org/materials/fullrecord.asp?name=ultramarine%20blue%2C%20synthetic synthetic ultramarine blue], and ceramic [http://cameo.mfa.org/materials/fullrecord.asp?name=glaze glaze]. It is also used as a leveling agent in dyeing textiles to ensure even color acceptance. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 51: | Line 51: | ||
== Authority == | == Authority == | ||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p.785 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
* ''The Dictionary of Paper'', American Paper Institute, New York, Fourth Edition, 1980 | * ''The Dictionary of Paper'', American Paper Institute, New York, Fourth Edition, 1980 | ||
| Line 59: | Line 59: | ||
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 | ||
| Line 65: | Line 65: | ||
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "thenardite." | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "thenardite." Encyclopædia Britannica. 2005. Encyclopædia Britannica Premium Service 7 Apr. 2005 . |
| − | * | + | * C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 |
| − | * | + | * John and Margaret Cannon, ''Dye Plants and Dyeing'', Herbert Press, London, 1994 |
| − | * | + | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 |
| − | * | + | * Website address 1 Comment: photographic chemicals at www.jetcity.com/~mrjones/chemdesc.htm |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 07:23, 24 July 2013
Description
White, hygroscopic crystals or powder. Anhydrous sodium sulfate occurs in nature as the mineral thenardite. Thenardite occurs most often as an evaporation product near salt lakes and playas, and is mined in arid regions of northern Africa, Siberia, Canada, and the western United States. Anhydrous sodium sulfate is used in the manufacture of kraft paper, paperboard, glass, synthetic ultramarine blue, and ceramic glaze. It is also used as a leveling agent in dyeing textiles to ensure even color acceptance.
Synonyms and Related Terms
anhydrous sodium sulfate; anhydrous sodium sulphate (Br.); thenardite; salt cake
Other Properties
Soluble in water, glycerol. Insoluble in ethanol.
Orthorhombic crystals with perfect cleavage in one direction.
Mineral thenardite is vitreous, translucent and produces a white streak.
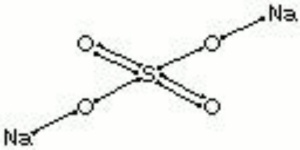
| Composition | Na2SO4 |
|---|---|
| CAS | 7757-82-6 |
| Mohs Hardness | 2.5-3.0 |
| Melting Point | 888 |
| Density | 2.671 |
| Molecular Weight | mol. wt. = 142.1 |
Hazards and Safety
Noncombustible.
LINK: International Chemical Safety Card
Additional Information
Grossi, "Acoustic emission monitoring to study sodium sulfate crystallization in monumental porous carbonate stone" Studies in Conservation 42 (1997), p. 115-125.
Authority
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p.785
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Dictionary of Paper, American Paper Institute, New York, Fourth Edition, 1980
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Encyclopedia Britannica, http://www.britannica.com Comment: "thenardite." Encyclopædia Britannica. 2005. Encyclopædia Britannica Premium Service 7 Apr. 2005 .
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- John and Margaret Cannon, Dye Plants and Dyeing, Herbert Press, London, 1994
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Website address 1 Comment: photographic chemicals at www.jetcity.com/~mrjones/chemdesc.htm