Difference between revisions of "Acetyl chloride"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 47: | Line 47: | ||
[http://www.cdc.gov/niosh/ipcsneng/neng0210.html International Chemical Safety Card] | [http://www.cdc.gov/niosh/ipcsneng/neng0210.html International Chemical Safety Card] | ||
| − | == | + | == Sources Checked for Data in Record == |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 | ||
Revision as of 12:52, 29 April 2016
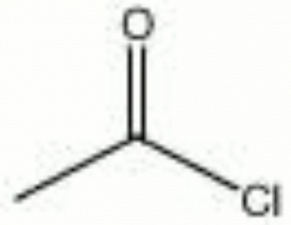
Description
A highly corrosive, fuming liquid with a pungent odor. Acetyl chloride is used as a reagent to detect the presence of Cholesterol and the presence of water in organic liquids.
Synonyms and Related Terms
chloride acetic acid; acetic chloride; ethanoyl chloride
Other Properties
Reacts violently with water.
Miscible in benzene, chloroform, ether.
| Composition | C2H3ClO |
|---|---|
| CAS | 75-36-5 |
| Melting Point | -112 |
| Density | 1.104 |
| Molecular Weight | mol. wt. = 78.50 |
| Refractive Index | 1.3898 |
| Boiling Point | 52 |
Hazards and Safety
Flammable. Flash point = 4C Corrosive. Contact, ingestion, and inhalation destroys tissues. Lachrymator.
Reacts violently with water. Incompatible with water, alcohols, oxidizing agents, strong bases. Produces toxic combustion products: carbon monoxide, hydrogen chloride, phosgene.
International Chemical Safety Card
Sources Checked for Data in Record
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983
- MSDS Sheet Comment: MFA, Conservation and Collections Management dept.