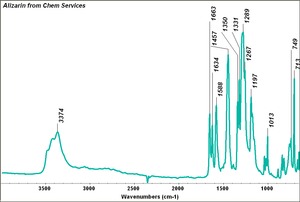
Alizarin, synthetic
Description
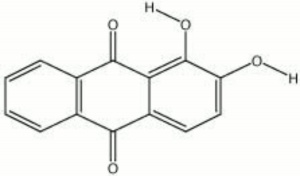
A synthetic form of alizarin (1,2-dihydroxyanthraquinone) was first made in 1868 by Carl Graebe and Carl Lieberman, from Anthracene, a coal tar product. Prior to that time, alizarin was obtained from the root of the madder plant, Rubia tinctorum L.. The orthorhombic, orange-red crystals of alizarin appear brownish-yellow in powder form. Alizarin is most commonly used for the derivatization of other dyes, but it is also known as a textile dye, a pigment, and an indicator. To prepare alizarin crimson pigment, alizarin is precipitated on a neutral base of Alumina trihydrate producing a brilliant, transparent color that has fair to good permanency. Alizarin will turn blue to purple in basic aqueous solutions, but it is more often used as an acid-base indicator in alcoholic solutions (0.5%) where it changes from yellow at pH 5.5 to red at pH 6.8. Alizarin is also used in spot tests as a reagent for [aluminum|aluminum]], Indium, Mercury, Zinc, and Zirconium. As a textile dye, the cloth (Cotton, Wool, or Silk) must be mordanted with a metal oxide. The shade produced depends on the metal present: aluminum yields a red; iron, a dark violet; and Chromium, a reddish-brown.
- See also [[http://cameo.mfa.org/wiki/Category:Uemura_dye_archive Uemera Dye Archive
Synonyms and Related Terms
1,2-dihydroxyanthraquinone; alizarine; Pigment Red 83 (calcium lake); Mordant Red 11; CI 58000; rouge d'alizarine (Fr.); Alizarin, synthetisch (Deut.); alizarina sintética (Esp.); alizarini, synthetiki (Gr.); alizarina sintetica (It.); alizarine (Ned.); alizarina, sintética (Port.); permanent violet; permanent crimson; alizarin crimson; crimson madder;
Physical and Chemical Properties
Soluble in aromatic solvents, acetone, hot methanol and ether.
Partially soluble in ethanol and water.
Alizarin does not fluoresce in ultraviolet light. Turns blue-purple in dilute alkaline solutions.
ISO R105 Lightfastness Classification = 4-5
| Composition | C14H6O2(OH)2 |
|---|---|
| CAS | 72-48-0 |
| Melting Point | 289-290 |
| Molecular Weight | mol. wt. = 240.22 |
| Boiling Point | 430 |
Hazards and Safety
Combustible. Slight potential for allergic reaction by contact, inhalation or ingestion.
ThermoFisher Scientific: SDS
Sources Checked for Data in Record
- Artists' Pigments: A Handbook of their History and Characteristics, Elisabeth West FitzHugh, Oxford University Press, Oxford, Vol. 3, 1997 Comment: H.Schweppe, J.Winter, "Madder and Alizarin"
- Encyclopedia Britannica, http://www.britannica.com Comment: "alizarin" [Accessed May 24, 2003]. Discovered 1868, first sold 1871
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 475
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing) Comment: Discovered 1868
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986 Comment: First appeared for sale in 1870
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Monona Rossol, The Artist's Complete Health and Safety Guide, Allworth Press, New York, 1994
- R.Feller, M.Curran, C.Bailie, 'Identification of Traditional Organic Colorants Employed in Japanese Prints and Determination of their Rates of Fading', Japanese Woodblock Prints, Allen Memorial Art Museum, Oberlin College, Oberlin, 1984 Comment: ISO rating
- A.Scharff, 'Synthetic dyestuffs for textiles and their fastness to washing', ICOM-CC Preprints Lyon, Getty Conservation Institute, Los Angeles, 1999. p.654-660.
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Website address: www.straw.com/sig/dyehist
- Pigments Through the Ages: http://webexhibits.org/pigments/indiv/technical/alizarin.html ; RI=1.70 alizarin, 1.66 madder lake;
- Colour Index International online at www.colour-index.org Comment: Structure; gives discoverer of calcium lake as Robequet & Colin 1826