Difference between revisions of "Amidol"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| (One intermediate revision by the same user not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | + | [[[SliderGallery rightalign|amidol.jpg~Chemical structure]]] | |
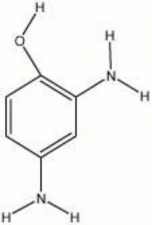
A colorless crystalline compound used as a photographic [[developer|developer]] since 1892. Amidol, or diaminophenol hydrochloride, is also used to dye [[fur|furs]] and [[hair|hair]]. | A colorless crystalline compound used as a photographic [[developer|developer]] since 1892. Amidol, or diaminophenol hydrochloride, is also used to dye [[fur|furs]] and [[hair|hair]]. | ||
| Line 7: | Line 7: | ||
acrol; diaminophenol hydrochloride; 2,4-diaminophenol dihydrochloride | acrol; diaminophenol hydrochloride; 2,4-diaminophenol dihydrochloride | ||
| − | + | == Physical and Chemical Properties == | |
| − | |||
| − | == | ||
| − | Turns dark brown with age. Produces difficult to remove stains on most materials. | + | * Turns dark brown with age. |
| + | * Produces difficult to remove stains on most materials. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 22: | Line 21: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 205 | + | | 205 C |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 28: | Line 27: | ||
|} | |} | ||
| − | == | + | == Risks == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | Fisher Scientific: [https://fscimage.fishersci.com/msds/95964.htm MSDS] | + | * Ingestion is toxic and may result in vertigo, convulsions and coma. |
| + | * Inhalation and contact cause irritation and may produce an allergic reaction. | ||
| + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/95964.htm MSDS] | ||
| − | == | + | == Physical and Chemical Properties == |
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| Line 44: | Line 41: | ||
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 | ||
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Amidol (Accessed Mar. 20, 2006) |
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
Latest revision as of 13:13, 26 April 2022
Description
A colorless crystalline compound used as a photographic Developer since 1892. Amidol, or diaminophenol hydrochloride, is also used to dye furs and Hair.
Synonyms and Related Terms
acrol; diaminophenol hydrochloride; 2,4-diaminophenol dihydrochloride
Physical and Chemical Properties
- Turns dark brown with age.
- Produces difficult to remove stains on most materials.
| Composition | C6H3(NH2)2OH-2HCl |
|---|---|
| CAS | 137-09-7 |
| Melting Point | 205 C |
| Molecular Weight | mol. wt.=197.07 |
Risks
- Ingestion is toxic and may result in vertigo, convulsions and coma.
- Inhalation and contact cause irritation and may produce an allergic reaction.
- Fisher Scientific: MSDS
Physical and Chemical Properties
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983
- Wikipedia: http://en.wikipedia.org/wiki/Amidol (Accessed Mar. 20, 2006)
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998