Difference between revisions of "Aragonite"
| (3 intermediate revisions by 3 users not shown) | |||
| Line 10: | Line 10: | ||
calcium carbonate; nacre; shell white; coral; Aragonit (Deut., Pol.); aragonita (Esp.); aragonito (Esp.); aragonite (Fr., Port.); aragoniet (Ned.) | calcium carbonate; nacre; shell white; coral; Aragonit (Deut., Pol.); aragonita (Esp.); aragonito (Esp.); aragonite (Fr., Port.); aragoniet (Ned.) | ||
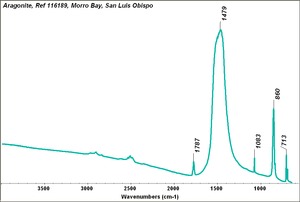
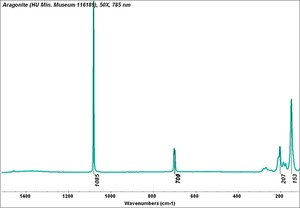
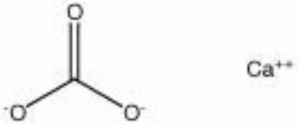
| − | [[[SliderGallery rightalign| | + | [[[SliderGallery rightalign|Aragonite 2.TIF~FTIR (MFA)|Aragonite (HU Min. Museum 116189), 50X, 785 nm copy.tif~Raman (MFA)|aragonite.jpg~Chemical structure]]] |
== Other Properties == | == Other Properties == | ||
| Line 49: | Line 49: | ||
== Additional Information == | == Additional Information == | ||
| − | ° R. Gettens, E. West Fitzhugh, R.Feller, "Calcium Carbonate Whites", ''Artists Pigments'', Vol. 2., A. Roy ed. Oxford University Press, Oxford, 1993. ° Mineralogy Database: [http://www.webmineral.com/data/Aragonite.shtml Aragonite] | + | ° R. Gettens, E. West Fitzhugh, R.Feller, "Calcium Carbonate Whites", ''Artists Pigments'', Vol. 2., A. Roy ed. Oxford University Press, Oxford, 1993. |
| + | |||
| + | ° Mineralogy Database: [http://www.webmineral.com/data/Aragonite.shtml Aragonite] | ||
== Additional Images == | == Additional Images == | ||
| Line 59: | Line 61: | ||
| − | == | + | == Sources Checked for Data in Record == |
* Jack Odgen, ''Jewellery of the Ancient World'', Rizzoli International Publications Inc., New York City, 1982 | * Jack Odgen, ''Jewellery of the Ancient World'', Rizzoli International Publications Inc., New York City, 1982 | ||
Revision as of 09:53, 14 November 2019
Description
A crystalline form of Calcium carbonate that occurs in Coral, shells, pearls, stalactites, and water deposits. Aragonite was named after the Aragon region in Spain where it was first discovered. Its orthorhombic system forms compact, acicular crystals that make it harder and heavier than Calcite. When aragonite is formed by water deposition of calcium carbonate, the crystals often grow in radiating flowers. Aragonite mines are located in Europe, Bolivia, and the U.S. (New Mexico, Arizona). Aragonite was used in antiquity for beads and decorative items. It can be converted to calcite with heat (470 C) and changes slowly to calcite at room temperature.
Synonyms and Related Terms
calcium carbonate; nacre; shell white; coral; Aragonit (Deut., Pol.); aragonita (Esp.); aragonito (Esp.); aragonite (Fr., Port.); aragoniet (Ned.)
Other Properties
Orthorhombic crystal system with platy or fibrous, acicular crystals that are often twinned. Reacts with acids to evolve carbon dioxide. Fluorescent. Brittle. Aragonite is harder and denser than calcite.
Luster = vitreous to resinous. Transparent to translucent. Fracture = subconchoidal. Streak = white
Strongly birefringent showing interference colors. Straight extinction
| Composition | CaCO3 |
|---|---|
| CAS | 471-34-1 |
| Mohs Hardness | 3.5 - 4.0 |
| Density | 2.93-2.95 |
| Molecular Weight | mol. wt. = 100.09 |
| Refractive Index | 1.530, 1.682, 1.686 |
Hazards and Safety
No significant hazards.
Mallinckrodt Baker: MSDS
Additional Information
° R. Gettens, E. West Fitzhugh, R.Feller, "Calcium Carbonate Whites", Artists Pigments, Vol. 2., A. Roy ed. Oxford University Press, Oxford, 1993.
° Mineralogy Database: Aragonite
Additional Images
Sources Checked for Data in Record
- Jack Odgen, Jewellery of the Ancient World, Rizzoli International Publications Inc., New York City, 1982
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Refractive Index: alpha=1.529-1.530; beta=1.680-1.682; gamma=1.685-1.686
- Encyclopedia Britannica, http://www.britannica.com Comment: aragonite" Encyclopædia Britannica [Accessed December 4, 2001
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Aragonite (Accessed Aug. 30 2005)
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 131
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998