Difference between revisions of "Cadmium yellow"
| (5 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
[[File:315 cad_yellow.jpg|thumb|Cadmium yellow]] | [[File:315 cad_yellow.jpg|thumb|Cadmium yellow]] | ||
== Description == | == Description == | ||
| + | [[File:Cdyellow C100x.jpg|thumb|Cadmium yellow at 100x; normal and UV light]] | ||
| + | A permanent, yellow pigment composed of [[cadmium sulfide]]. Cadmium yellows were synthetically prepared in Germany by Friedrich Strohmeyer in 1817. The bright yellow pigments slowly began to be used as artist paints in the mid 1840s, gaining in popularity in the early 20th century. Variations in particle size and chemical composition produce as range of colors from light yellow to orange. In the 1920s, the cadmium pigments were co-precipitated with [[barium sulfate]] to form the cheaper cadmium lithopone (cadmopone) pigments. Cadmium sulfide also occurs in minor amounts in the mineral greenockite. | ||
| − | |||
| − | |||
| − | |||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
cadmium sulfide; Pigment Yellow 37; CI 77191; Kadmiumgelb (Deut.); jaune de cadmium (Fr.); cadmium sulphide (Br.); giallo di cadmio (It.); amarillo de cadmio (Esp.); kitrino toy kadmioy (Gr.); cadmiumgeel (Ned.); amarelo de cádmio (Port.); cadmium lithopone; cadmopone; Aurora yellow; daffodil; radiant yellow; cadmia; Orient yellow; jaune brilliant; Cadmolith | cadmium sulfide; Pigment Yellow 37; CI 77191; Kadmiumgelb (Deut.); jaune de cadmium (Fr.); cadmium sulphide (Br.); giallo di cadmio (It.); amarillo de cadmio (Esp.); kitrino toy kadmioy (Gr.); cadmiumgeel (Ned.); amarelo de cádmio (Port.); cadmium lithopone; cadmopone; Aurora yellow; daffodil; radiant yellow; cadmia; Orient yellow; jaune brilliant; Cadmolith | ||
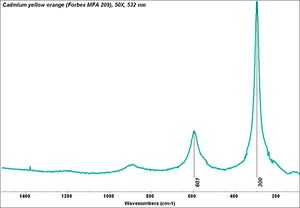
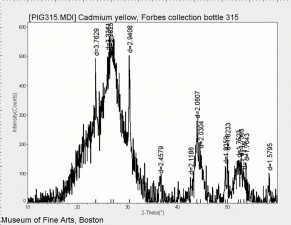
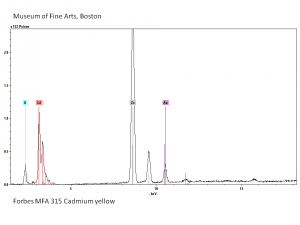
| − | [[[SliderGallery rightalign|PIG315.jpg~XRD|f315sem.jpg~SEM|f315edsbw.jpg~EDS|Slide25 FC315.PNG~XRF]]] | + | [[[SliderGallery rightalign|Cadmium yellow orange (Forbes MFA 209), 50X, 532 nm copy.jpg~Raman (MFA)|PIG315.jpg~XRD|f315sem.jpg~SEM|f315edsbw.jpg~EDS|Slide25 FC315.PNG~XRF]]] |
| − | + | == Risks == | |
| − | == | ||
| − | + | * Toxic by ingestion and inhalation. Carcinogen. | |
| + | * M.Graham and Co. Watercolor: [https://mgraham.com/wp-content/uploads/2017/09/Graham-Watercolor-SDS.pdf SDS] | ||
| + | == Physical and Chemical Properties == | ||
| − | The tiny yellow particles (about 1 micrometer) have a high refractive index. | + | * Cubic or hexagonal crystals. |
| + | * Soluble in concentrated mineral acids with the evolution of H2S. Insoluble in water. | ||
| + | * May fluoresce red. | ||
| + | * The tiny yellow particles (about 1 micrometer) have a high refractive index. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 23: | Line 26: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 4.35 | + | | 4.35 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 34: | ||
| e=2.506, w=2.529 | | e=2.506, w=2.529 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Additional Images == | == Additional Images == | ||
<gallery> | <gallery> | ||
| − | File:14_Cadmium_yellow_500X.jpg|Cadmium yellow | + | File:14_Cadmium_yellow_500X.jpg|Cadmium yellow at 500x |
| − | File:14_Cadmium_yellow_500X_pol.jpg|Cadmium yellow | + | File:14_Cadmium_yellow_500X_pol.jpg|Cadmium yellow at 500x in polarized light |
</gallery> | </gallery> | ||
| + | ==Resources and Citations== | ||
| + | * I. Fiedler, M. Bayard, "Cadmium yellows, oranges and reds", ''Artists Pigments'', Volume 1, R. Feller (ed.), Cambridge University Press: Cambridge, 1986. | ||
| − | + | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004. | |
| − | |||
| − | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | ||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density = 4.35 and ref. index = 2.35-2.48 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density = 4.35 and ref. index = 2.35-2.48 | ||
| Line 56: | Line 51: | ||
* Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | ||
| − | * | + | * Pigments Through the Ages: http://webexhibits.org/pigments/indiv/technical/cdyellow.html - e=2.506, w=2.529 |
* Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 | * Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 | ||
Latest revision as of 14:59, 11 May 2022
Description
A permanent, yellow pigment composed of Cadmium sulfide. Cadmium yellows were synthetically prepared in Germany by Friedrich Strohmeyer in 1817. The bright yellow pigments slowly began to be used as artist paints in the mid 1840s, gaining in popularity in the early 20th century. Variations in particle size and chemical composition produce as range of colors from light yellow to orange. In the 1920s, the cadmium pigments were co-precipitated with Barium sulfate to form the cheaper cadmium lithopone (cadmopone) pigments. Cadmium sulfide also occurs in minor amounts in the mineral greenockite.
Synonyms and Related Terms
cadmium sulfide; Pigment Yellow 37; CI 77191; Kadmiumgelb (Deut.); jaune de cadmium (Fr.); cadmium sulphide (Br.); giallo di cadmio (It.); amarillo de cadmio (Esp.); kitrino toy kadmioy (Gr.); cadmiumgeel (Ned.); amarelo de cádmio (Port.); cadmium lithopone; cadmopone; Aurora yellow; daffodil; radiant yellow; cadmia; Orient yellow; jaune brilliant; Cadmolith
Risks
- Toxic by ingestion and inhalation. Carcinogen.
- M.Graham and Co. Watercolor: SDS
Physical and Chemical Properties
- Cubic or hexagonal crystals.
- Soluble in concentrated mineral acids with the evolution of H2S. Insoluble in water.
- May fluoresce red.
- The tiny yellow particles (about 1 micrometer) have a high refractive index.
| Composition | CdS |
|---|---|
| Density | 4.35 g/ml |
| Molecular Weight | 144.48 |
| Refractive Index | e=2.506, w=2.529 |
Additional Images
Resources and Citations
- I. Fiedler, M. Bayard, "Cadmium yellows, oranges and reds", Artists Pigments, Volume 1, R. Feller (ed.), Cambridge University Press: Cambridge, 1986.
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004.
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density = 4.35 and ref. index = 2.35-2.48
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Pigments Through the Ages: http://webexhibits.org/pigments/indiv/technical/cdyellow.html - e=2.506, w=2.529
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: 'Pigments'
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000