Difference between revisions of "Calcite"
| Line 14: | Line 14: | ||
== Other Properties == | == Other Properties == | ||
| − | Rhombohedral crystal system. Perfect cleavage in three directions. Fracture = conchoidal. High birefringence. Can doubly refract light. Aged surfaces of calcite may | + | Rhombohedral crystal system. Perfect cleavage in three directions. Fracture = conchoidal. High birefringence. Can doubly refract light. Aged surfaces of calcite may fluoresces purple or blue in ultraviolet light; freshly cleaved surfaces are white to yellow. Reacts with acids to evolve carbon dioxide. Luster = vitreous. Streak = white. |
{| class="wikitable" | {| class="wikitable" | ||
Revision as of 10:07, 25 July 2013
Description
The most common crystalline form of calcium carbonate. Calcite is widely distributed throughout the world as chalk, limestone, and marble. Major deposits were formed from ancient sea beds and hard water deposits (e.g., stalactites). Iceland is famous for producing large clear birefractive calcite crystals that are used in optical systems (Iceland spar). In addition to clear colorless crystals, calcite may also appear white or pale shades of other colors depending on the crystal size and the presence of impurities. Marble is limestone that has been metamorphosed to form compact crystals of calcite. Calcite has been gathered or mined since Paleolithic times. In the form of chalk, calcite was powdered for use as a pigment and as an ingredient in the manufacture of steel, cement, and glass. Limestone and marble are used for sculpture and buildings.
Synonyms and Related Terms
calcium carbonate; marble; limestone; Iceland spar; dogtooth spar; dog-tooth spar; nailhead spar; satin spar; calcareous spar; calc spar; calcspar; calcite (It., Fr., Port.); Kalzit, Calcit (Deut.); calcita (Esp.); calciet (Ned.); calcium salt of carbonic acid; Pigment White 18; CI 7720
Other Properties
Rhombohedral crystal system. Perfect cleavage in three directions. Fracture = conchoidal. High birefringence. Can doubly refract light. Aged surfaces of calcite may fluoresces purple or blue in ultraviolet light; freshly cleaved surfaces are white to yellow. Reacts with acids to evolve carbon dioxide. Luster = vitreous. Streak = white.
| Composition | CaCO3 |
|---|---|
| CAS | 471-34-1 |
| Mohs Hardness | 3.0 |
| Density | 2.71-2.72 |
| Molecular Weight | mol. wt. = 100.09 |
| Refractive Index | 1.486; 1.658; (1.566) |
Hazards and Safety
International Chemical Safety Card
Additional Information
° R. Gettens, E. West Fitzhugh, R.Feller, "Calcium Carbonate Whites", Artists Pigments, Vol. 2., A. Roy ed. Oxford University Press, Oxford, 1993. ° Mineralogy Database: Calcite
Comparisons
Properties of Common Abrasives
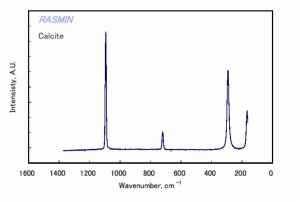
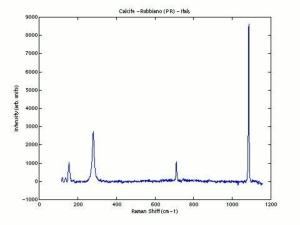
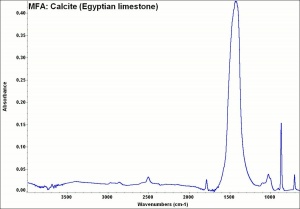
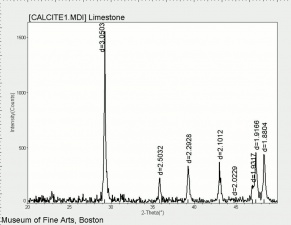
Additional Images
Authority
- Thomas Gregory, The Condensed Chemical Dictionary, Reinhold Publishing, New York, 3rd ed., 1942 Comment: Mohs =2
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 Comment: Mohs=3
- Encyclopedia Britannica, http://www.britannica.com Comment: "calcite" Encyclopædia Britannica [Accessed December 4, 2001]
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 138, Mohs=3
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Calcite (Accessed Sept 2 2005; hardness = 3, sp = 2.7
- Jack Odgen, Jewellery of the Ancient World, Rizzoli International Publications Inc., New York City, 1982
- A.Lucas, J.R.Harris, Ancient Egyptian Materials and Industries, Edward Arnold Publishers Ltd., London, 4th edition, 1962