Difference between revisions of "Calcium formate"
Jump to navigation
Jump to search
(username removed) |
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
||
| (One intermediate revision by one other user not shown) | |||
| Line 37: | Line 37: | ||
Fisher Scientific: [https://fscimage.fishersci.com/msds/97124.htm MSDS] | Fisher Scientific: [https://fscimage.fishersci.com/msds/97124.htm MSDS] | ||
| − | == | + | == Sources Checked for Data in Record == |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
Revision as of 14:22, 29 April 2016
Description
Colorless crystals or powder. Calcium formate is used as a preservative, as a buffer (pH 4) and in mineral tanning solutions.
Synonyms and Related Terms
calcium diformate; Calcoform
Other Properties
Orthorhombic crystals. Slowly soluble in hot water (pH = 4). Insoluble in ethanol.
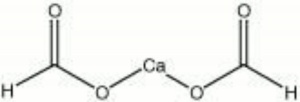
| Composition | CaC2H2O4 |
|---|---|
| CAS | 544-17-2 |
| Melting Point | 300 |
| Density | 2.02 |
| Molecular Weight | mol. wt. = 130.11 |
Hazards and Safety
Ingestion, inhalation, and contact cause irritation.
Fisher Scientific: MSDS
Sources Checked for Data in Record
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1642