Difference between revisions of "Calcium hydroxyapatite"
Jump to navigation
Jump to search
| Line 8: | Line 8: | ||
tribasic calcium phosphate; tricalcium orthophosphate; tricalcium phosphate; bone ash | tribasic calcium phosphate; tricalcium orthophosphate; tricalcium phosphate; bone ash | ||
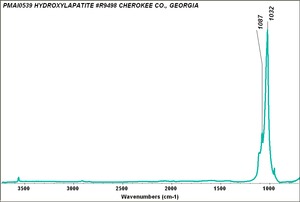
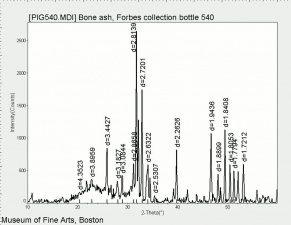
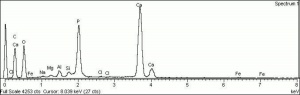
| + | [[[SliderGallery rightalign|Hydroxyapatite PMA.TIF~FTIR (PMA)|PIG540.jpg~XRD|f540sem.jpg~SEM|f540edsbw.jpg~EDS]]] | ||
| + | |||
| + | == Risks == | ||
| − | + | Nonflammable. | |
| − | == | + | Merz: [https://www.merzusa.com/wp-content/uploads/SF7806-05.pdf SDS] |
| + | == Physical and Chemical Properties == | ||
Soluble in mineral acids. Insoluble in water, ethanol, acetic acid. | Soluble in mineral acids. Insoluble in water, ethanol, acetic acid. | ||
| Line 35: | Line 39: | ||
| 1.63 | | 1.63 | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Sources Checked for Data in Record == | == Sources Checked for Data in Record == | ||
Revision as of 11:16, 29 August 2020
Description
A white, odorless, tasteless powder. Bone is primarily composed of calcium hydroxyapatite mixed with Calcium carbonate and organic binders. Calcium hydroxyapatite is used in the manufacture of Milk glass, as a polishing powder, as a mordant and as a buffer.
See also Apatite.
Synonyms and Related Terms
tribasic calcium phosphate; tricalcium orthophosphate; tricalcium phosphate; bone ash
Risks
Nonflammable.
Merz: SDS
Physical and Chemical Properties
Soluble in mineral acids. Insoluble in water, ethanol, acetic acid.
| Composition | Ca10(PO4)6(OH)2 |
|---|---|
| CAS | 1306-06-5 |
| Melting Point | 1670 |
| Density | 3.18 |
| Molecular Weight | mol. wt. = 1004.69 |
| Refractive Index | 1.63 |
Sources Checked for Data in Record
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1741