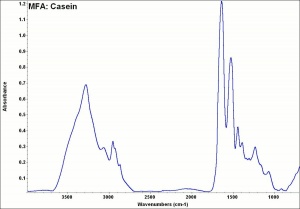
Difference between revisions of "Casein"
(username removed) |
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A natural [ | + | A natural [[phosphorus]]-containing [[protein]] found in [[milk]]. Casein is composed of the following major amino acids: [[glutamic acid]] (20.2%), proline (13.2%), aspartic acid (6.1%), [[leucine]] (9.0%), [[lysine]] (6.7%), valine (7.2%), tyrosine (5.5%), isoleucine (6.0%), and phenylalanine (5.1%) with no measurable amounts of [[hydroxyproline]] (Mills and White 1994). It has been used as a [[glue]] and binder since earliest recorded periods. Casein curds form naturally as milk sours, but it is also precipitated by adding dilute [[hydrochloric acid]] to hot skim milk. The curds are collected, washed and dried to form a white to yellowish powder. The dried casein is insoluble in water and alcohol but is soluble in carbonates and other alkaline solutions. For use, casein is soaked overnight in a solution with a weak alkali ([[ammonium carbonate]], [[borax]], or [[lime]]) to form a clear, viscous solution. Solutions of casein are used as [[casein adhesive|adhesives]] and as [[casein paint|paint]] binders in casein colors and architectural paints. In the late 19th century, casein was made into a plastic by treatment with formaldehyde. [[casein plastic|Casein plastics]] were used for small items such as buttons, beads, buckles, combs, cutlery handles, and knitting needles. They were often pigmented to simulate [[ivory]], [[horn]], or [[tortoiseshell]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | causeum (Lat.); | + | causeum (Lat.); caséine (Fr.); caseína (Esp., Port.); caseina (It.); caseinate; whey glue; milk acid powder; milk albumin; milk albumen; kasein; ammonium casein; borax casein; lime-casein; |
Examples include: Lactilith; Galalith; Erinoid; Kyloid; Casco Glue; | Examples include: Lactilith; Galalith; Erinoid; Kyloid; Casco Glue; | ||
| Line 27: | Line 27: | ||
== Additional Information == | == Additional Information == | ||
| − | K.Wehlte, ''The Materials and Techniques of Painting'', Van Nostrand Reinhold Co., New York, 1975 | + | ° K.Wehlte, ''The Materials and Techniques of Painting'', Van Nostrand Reinhold Co., New York, 1975. |
| − | + | ° J.S. Mills, R.White, ''The Organic Chemistry of Museum Objects'', Butterworth Heinemann, London, 1994. | |
| − | + | == Sources Checked for Data in Record == | |
| − | * | + | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: specific gravity = 1.259 |
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 152 |
| − | * | + | * Reed Kay, ''The Painter's Guide To Studio Methods and Materials'', Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983 |
| − | * | + | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) |
| − | * | + | * Tom Rowland, Noel Riley, ''A-Z Guide to Cleaning, Conserving and Repairing Antiques'', Constable and Co., Ltd., London, 1981 |
| − | * | + | * Boise Cascade Paper Group, ''The Paper Handbook'', Boise Cascade, Portland OR, 1989 |
| + | |||
| + | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 | ||
* ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | * ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | ||
| − | * | + | * Book and Paper Group, ''Paper Conservation Catalog'', AIC, 1984, 1989 |
| − | * | + | * Kurt Wehlte, ''The Materials and Techniques of Painting'', Van Nostrand Reinhold Co., New York, 1975 |
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
| − | * | + | * Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 14:37, 29 April 2016
Description
A natural Phosphorus-containing Protein found in Milk. Casein is composed of the following major amino acids: Glutamic acid (20.2%), proline (13.2%), aspartic acid (6.1%), Leucine (9.0%), Lysine (6.7%), valine (7.2%), tyrosine (5.5%), isoleucine (6.0%), and phenylalanine (5.1%) with no measurable amounts of Hydroxyproline (Mills and White 1994). It has been used as a Glue and binder since earliest recorded periods. Casein curds form naturally as milk sours, but it is also precipitated by adding dilute Hydrochloric acid to hot skim milk. The curds are collected, washed and dried to form a white to yellowish powder. The dried casein is insoluble in water and alcohol but is soluble in carbonates and other alkaline solutions. For use, casein is soaked overnight in a solution with a weak alkali (Ammonium carbonate, Borax, or Lime) to form a clear, viscous solution. Solutions of casein are used as adhesives and as paint binders in casein colors and architectural paints. In the late 19th century, casein was made into a plastic by treatment with formaldehyde. Casein plastics were used for small items such as buttons, beads, buckles, combs, cutlery handles, and knitting needles. They were often pigmented to simulate Ivory, Horn, or Tortoiseshell.
Synonyms and Related Terms
causeum (Lat.); caséine (Fr.); caseína (Esp., Port.); caseina (It.); caseinate; whey glue; milk acid powder; milk albumin; milk albumen; kasein; ammonium casein; borax casein; lime-casein;
Examples include: Lactilith; Galalith; Erinoid; Kyloid; Casco Glue;
Other Properties
Insoluble in water or ethanol when dry. Soluble in strong alkalis and ammonium hydroxide. Impervious to most modern paint strippers.
| Density | 1.259 (dry) |
|---|
Hazards and Safety
May yellow with time. Susceptible to microbiological attack.
Additional Information
° K.Wehlte, The Materials and Techniques of Painting, Van Nostrand Reinhold Co., New York, 1975.
° J.S. Mills, R.White, The Organic Chemistry of Museum Objects, Butterworth Heinemann, London, 1994.
Sources Checked for Data in Record
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: specific gravity = 1.259
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 152
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Tom Rowland, Noel Riley, A-Z Guide to Cleaning, Conserving and Repairing Antiques, Constable and Co., Ltd., London, 1981
- Boise Cascade Paper Group, The Paper Handbook, Boise Cascade, Portland OR, 1989
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Kurt Wehlte, The Materials and Techniques of Painting, Van Nostrand Reinhold Co., New York, 1975
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988