Difference between revisions of "Cellulose nitrate"
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | Some of the earliest synthetic resins were made from cellulose fibers. Cellulose nitrate was discovered by Henri Braconnot in | + | Some of the earliest synthetic resins were made from cellulose fibers. Cellulose nitrate was initially discovered by Henri Braconnot in 1833 (he named the compound xyloidine) and again in 1838 by Théophile-Jules Pelouze (he named the compound nitrimidine) when each man combined cellulose with nitric acid. The material was patented in 1846 by two other chemists and was initially used as an explosive (gun cotton). Nitrocellulose was developed for use as a plastic, when the chemistry was modified to control the degree of substitution (number of nitro groups on the chain). It was marketed in the U.S. as [[Celluloid]], a proprietary mixture of cellulose nitrate with [[camphor]] added as a plasticizer. Celluloid was molded into numerous shapes such as piano keys, billiard balls, ping pong balls, dolls, buttons and boxes. It was used to make inexpensive objects and decorations that imitated the appearance of [[ivory]], [[amber]], [[carnelian]], [[coral]], [[seashell]] and [[tortoiseshell]]. Cellulose nitrate was also used for photographic film (from the 1880s to 1920s) and animated drawing cels. Because of its instability, its use for cinematography was limited in 1912 and banned in 1951. In the early 20th century, cellulose nitrate was often used for clear lacquers, fabric dopes, adhesives, and high-gloss paints. During the 1940s and 50s, cellulose nitrate was commercially sold in mixed with natural resins ([[dammar]], [[shellac]], [[copal]], etc) as a waterproof varnish. Cellulose nitrate is inherently unstable and slowly decomposes at room temperature. Ultraviolet light, heat, and/or high humidities hasten its decomposition. Cellulose nitrate is still sold in adhesives, coatings, and explosives. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Revision as of 10:46, 5 July 2020
Description
Some of the earliest synthetic resins were made from cellulose fibers. Cellulose nitrate was initially discovered by Henri Braconnot in 1833 (he named the compound xyloidine) and again in 1838 by Théophile-Jules Pelouze (he named the compound nitrimidine) when each man combined cellulose with nitric acid. The material was patented in 1846 by two other chemists and was initially used as an explosive (gun cotton). Nitrocellulose was developed for use as a plastic, when the chemistry was modified to control the degree of substitution (number of nitro groups on the chain). It was marketed in the U.S. as Celluloid, a proprietary mixture of cellulose nitrate with Camphor added as a plasticizer. Celluloid was molded into numerous shapes such as piano keys, billiard balls, ping pong balls, dolls, buttons and boxes. It was used to make inexpensive objects and decorations that imitated the appearance of Ivory, Amber, Carnelian, Coral, Seashell and Tortoiseshell. Cellulose nitrate was also used for photographic film (from the 1880s to 1920s) and animated drawing cels. Because of its instability, its use for cinematography was limited in 1912 and banned in 1951. In the early 20th century, cellulose nitrate was often used for clear lacquers, fabric dopes, adhesives, and high-gloss paints. During the 1940s and 50s, cellulose nitrate was commercially sold in mixed with natural resins (Dammar, Shellac, Copal, etc) as a waterproof varnish. Cellulose nitrate is inherently unstable and slowly decomposes at room temperature. Ultraviolet light, heat, and/or high humidities hasten its decomposition. Cellulose nitrate is still sold in adhesives, coatings, and explosives.
Synonyms and Related Terms
nitrocellulose; Zellulosenitrat (Deut.); nitrocellulose (Fr.); fulmicoton (Fr.); nitrocellulosa (It.); schietkatoen (Ned.); cellulosenitraat (Ned.); nitroceluloza (Pol.); nitrato de celulosa (Esp.); nitrocelulosa (Esp.); nitrato di cellulosa, celluloide (It.); nitrocelulose (Port.); trinitrocelulose (Port.); algodão-pólvora (Port.); bomullskrut (Sven.); nitrocellulosakrut (Sven.); nitrerad cellulosa (Sven.); nitro-cellulose; chardonnet; French ivory; pyroxylin; airplane wing dope, guncotton; gun-cotton; collodion; celloidin; celluidine; nitrocotton; photoxylin
Examples: Celluloid; Xyloidine; Nitramidine; Parkesine; Zapon-lack [Dulux]; HMG [H.Marcel Guest]; Durofix [Rawplug]; Duco® cement [DuPont]; UHU Hart [Linger & Fischer, Germany];
Applications
Risks
Highly flammable. Explosion risk. Flash point = 13C (55F)
Ultraviolet light, high temperatures and moisture accelerate degradation. May adversely react with metals (lead, silver, tin, iron, copper and zinc).
Physical and Chemical Properties
Birefringent. Softening point 155-200C. No electical charge when rubbed with silk.
Soluble in ketones, esters, and ether alcohol mixtures. Insoluble in water, ethanol, and hydrocarbons. Burns with a bright, violent flame; smells of nitrogen oxides.
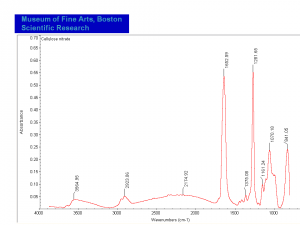
One drop of diphenylamine solution (6% in conc. sulfuric acid) gives positive deep blue color for cellulose nitrate
| Density | 1.34-1.40 |
|---|---|
| Refractive Index | 1.49-1.51 |
Additional Information
° J.Reilly, "Celluloid Objects: Their Chemistry and Preservation" JAIC, 145-162, 1991. Link
° Museum Handbook, Part 1. Museums Collections. Web Edition. Appendix M. Management of Cellulose Nitrate and Cellulose Acetate Films, NPS, 2001. http://www.cr.nps.gov/museum/publications/MHI/AppendM.pdf
Links to Oddy Test results posted on AIC Wiki Materials Database Pages for individual materials below
Cellulose Nitrate tested in 2013
Comparisons
General Characteristics of Polymers
Physical Properties for Selected Thermoplastic Resins
Sources Checked for Data in Record
- M.Kaufman, The First Century of Plastics, The Plastics and Rubber Institute, London, 1963
- History of Plastics: www.nswpmith.com.au/historyofplastics.html
- Wikipedia: http://en.wikipedia.org/wiki/Cellulose_nitrate (Accessed Jan. 15, 2006) - discovered by Henri Braconnot, a French chemist in 1832
- B. Gascoigne, How to Identify Prints, Thames & Hudson, London, 2004
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 171
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- C.V.Horie, Materials for Conservation, Butterworth-Heineman, London, 1997
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- S.R.Trotman, E.R. Trotman, Textile Analysis, J.B. Lippincott Company, Philadelphia, 1932
- Tom Rowland, Noel Riley, A-Z Guide to Cleaning, Conserving and Repairing Antiques, Constable and Co., Ltd., London, 1981
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Plastic"; "Ivory"
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988