Difference between revisions of "Chlorpyrifos"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 10: | Line 10: | ||
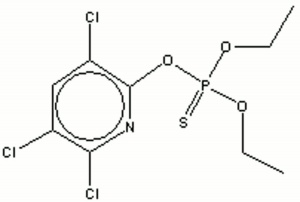
[[[SliderGallery rightalign|chlorpyrifosir.jpg~FTIR|chlorpyrifosstr.jpg~Chemical structure]]] | [[[SliderGallery rightalign|chlorpyrifosir.jpg~FTIR|chlorpyrifosstr.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | Toxic by inhalation, ingestion and skin contact. Corrosive to iron, copper and brass. Discolors red dyes. May leave deposits on nearby surfaces. Combustible. | ||
| + | |||
| + | Cayman Chemical [https://www.caymanchem.com/msdss/21412m.pdf SDS] | ||
| + | |||
| + | == Physical and Chemical Properties == | ||
Soluble in acetone, benzene, carbon disulfide, carbon tetrachloride, chloroform, diethyl ether, methylene chloride, xylene. Insoluble in water. | Soluble in acetone, benzene, carbon disulfide, carbon tetrachloride, chloroform, diethyl ether, methylene chloride, xylene. Insoluble in water. | ||
| Line 32: | Line 38: | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | == | + | == Resources and Citations == |
* Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
Revision as of 09:24, 11 August 2020
Description
Toxic, white granule crystals with a strong odor. Chlorpyrifos is an organophosphate type contact Insecticide sold under the brand name of Dursban. Dursban is broad spectrum insecticide used to control cockroaches, chinch bugs, fleas, termites, ants, and ticks. It has been used in pet flea and tick collars.
Synonyms and Related Terms
chlorpyriphosethyl; chloropyrifos (sp); O,O-diethyl O-3,5,6-trichloro-2-pyridyl phosphorothioate; Dursban [Dow]: Lorsban; Dowco 179; ENT 27311
Risks
Toxic by inhalation, ingestion and skin contact. Corrosive to iron, copper and brass. Discolors red dyes. May leave deposits on nearby surfaces. Combustible.
Cayman Chemical SDS
Physical and Chemical Properties
Soluble in acetone, benzene, carbon disulfide, carbon tetrachloride, chloroform, diethyl ether, methylene chloride, xylene. Insoluble in water.
| Composition | C9H11Cl3NO3PS |
|---|---|
| CAS | 2921-88-2 |
| Melting Point | 41-42 |
| Density | 1.398 |
| Molecular Weight | mol. wt. = 350.6 |
Resources and Citations
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983
- Lynda A. Zycherman, J.Richard Schrock, A Guide to Museum Pest Control, FAIC and Association of Systematics Collections, Washington DC, 1988
- J. Dawson, CCI Technical Bulletin, 'Solving Museum Insect Problems: Chemical Control' , Canadian Conservation Institute, Ottawa, No. 15
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002