Difference between revisions of "Chrome orange"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 52: | Line 52: | ||
H. Kuhn, M.Curran, "Chrome Yellow and Other Chromate Pigments", ''Artists Pigments'', Volume 1, R. Feller (ed.), Cambridge University Press: Cambridge, 1986. | H. Kuhn, M.Curran, "Chrome Yellow and Other Chromate Pigments", ''Artists Pigments'', Volume 1, R. Feller (ed.), Cambridge University Press: Cambridge, 1986. | ||
| − | == | + | == Sources Checked for Data in Record == |
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
Revision as of 14:57, 29 April 2016
Description
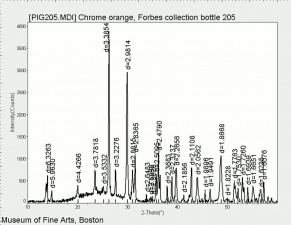
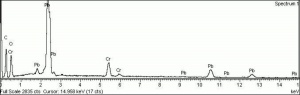
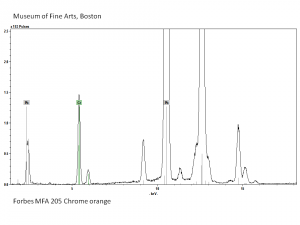
A basic lead chromate pigment that ranges in shades from orange to red. First made in 1809 by Vauquelin, basic lead chromate is formed by heating lead chromate and sodium hydroxide solution. The color of chrome red, or chrome orange can vary from a brown-yellow to a brick-red color depending on particle size and to the ratio of lead oxide to lead chromate. It is stable in light but is not widely used because of its sensitivity to sulfur gases. Most often, chrome red is used as an anticorrosive base coats for steel. See also Chrome yellow.
Synonyms and Related Terms
basic lead (II) chromate; Pigment Orange 21 and 45; CI 77601; phoenicochroite (mineral); anaranjado de cromo (Esp.); Chromorange (Deut.); chromorange (Fr.); portokali toy chromioy (Gr.); arancio cromo (It.); chroomoranje (Ned.); laranja de crómio (Port.); orange paste; American vermilion; plumbous chromate
Other Properties
Soluble in strong acids and alkalis. Insoluble in water. Can change to yellow in acetic acid.
| Composition | PbO-CrO3 |
|---|---|
| CAS | 7758-97-6 |
| Mohs Hardness | 2.5 |
| Melting Point | 844 |
| Density | 6.3-6.7 |
| Molecular Weight | mol. wt. = 323.2 |
| Refractive Index | 2.42; 2.7; 2.7 |
Hazards and Safety
Toxic by inhalation or ingestion. Skin contact may cause irritation or ulcers.
Carcinogen, teratogen, suspected mutagen.
Discolored by heat and sulfur fumes
LINK: International Chemical Safety Card
Additional Information
H. Kuhn, M.Curran, "Chrome Yellow and Other Chromate Pigments", Artists Pigments, Volume 1, R. Feller (ed.), Cambridge University Press: Cambridge, 1986.
Sources Checked for Data in Record
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5423
- Website address 1 Comment: http://webexhibits.org/pigments/indiv/overview/crorange.html