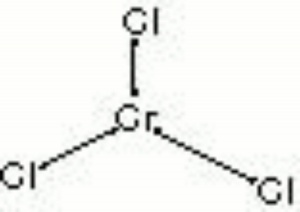
Chromic chloride
Jump to navigation
Jump to search
Description
Shiny, violet, platelike crystals. Chromic chloride reacts slowly with water to form a hexahydrate form that is a dark green, water soluble salt. Chromium chloride is used for tanning leathers and for mordanting dyes on textiles. It is also used for vapor plating Chromium and as a corrosion inhibitor.
Synonyms and Related Terms
chromium chloride; chromium trichloride; chromium sesquichloride
Other Properties
Insoluble in water, ethanol
| Composition | CrCl3 |
|---|---|
| CAS | 10025-73-7 |
| Melting Point | 1152 |
| Density | 2.87 |
| Molecular Weight | mol. wt. = 158.35 |
| Boiling Point | 1300(dec) |
Hazards and Safety
Toxic.
Mallinckrodt Baker: MSDS
Sources Checked for Data in Record
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry # 2278
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 797