Difference between revisions of "Cinnabar"
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| (6 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
[[File:Cinnabaremr1.jpg|thumb|Cinnabar]] | [[File:Cinnabaremr1.jpg|thumb|Cinnabar]] | ||
== Description == | == Description == | ||
| + | [[File:154 cinnabar.jpg|thumb|Cinnabar]] | ||
| + | A soft, dense, red, native ore composed of [[Mercuric sulfide, red|mercuric sulfide]]. Cinnabar is widely distributed around the world and is most often found in veins near volcanic rocks or hot springs. It has been mined from the Spanish cliffs near Almadén for over 2000 years. Other deposits are found in Italy (Iudrio), Germany, Austria, Yugoslavia, China (Hunan, Kweichow), Turkestan, Mexico, Peru (Huancavelica) and the United States (Texas, California, Nevada). When finely ground cinnabar produces a bright red opaque pigment that has been used since antiquity. Red mercuric sulfide has been made made synthetically since at least the 8th century in Europe and possibly earlier in China. Synthetic mercuric sulfide is called [[vermilion]]. Due to impurities, vermilion was favored over ground cinnabar as a red pigment. When exposed to ultraviolet light, mercuric sulfide darkens as a portion changes from its normal red crystalline form to a black isomorph. This can result in splotchy discolorations. | ||
| − | + | [[File:cinnabar C100x.jpg|thumb|Cinnabar at 100x (visible light on left, UV light on right)]] | |
| − | |||
| − | [[File: | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 10: | Line 10: | ||
red mercuric sulfide; Pigment Red 106; CI 77766; Zinnober (Deut.); Zinnoberersatz (Ned.); cinabrio (Esp.); cinabre (Fr.); cinabro (It.); cinábrio (Port.); cinnaber (Ned.); shinsha (Jap.); tan-sha (Chin.); sulfure de mercure (Fr.); Chinese vermilion; English vermilion; vermilion (synthetic pigment); red sulfuretted oxide of mercury; liver ore | red mercuric sulfide; Pigment Red 106; CI 77766; Zinnober (Deut.); Zinnoberersatz (Ned.); cinabrio (Esp.); cinabre (Fr.); cinabro (It.); cinábrio (Port.); cinnaber (Ned.); shinsha (Jap.); tan-sha (Chin.); sulfure de mercure (Fr.); Chinese vermilion; English vermilion; vermilion (synthetic pigment); red sulfuretted oxide of mercury; liver ore | ||
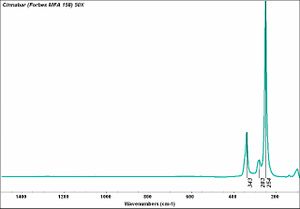
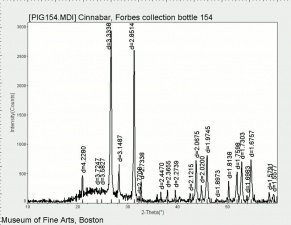
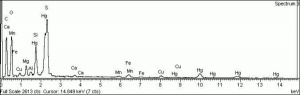
| − | [[[SliderGallery rightalign| | + | [[[SliderGallery rightalign|Cinnabar (Forbes MFA 158) 50X (640x445).jpg~Raman (MFA)|PIG154.jpg~XRD|f154sem.jpg~SEM|f154edsbw.jpg~EDS]]] |
| − | + | == Risks == | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | = | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * Highly toxic by ingestion, inhalation and skin absorption. | |
| + | * Darkens in ultraviolet light. | ||
| + | * Kremer-Pigmente: [https://www.kremer-pigmente.com/elements/resources/products/files/10620_MSDS.pdf MSDS] | ||
| − | + | ==Physical and Chemical Properties== | |
| − | + | * Composition = HgS | |
| − | = | + | * Soluble in aqua regia. Unaffected by alkalis. |
| − | + | * Hexagonal crystal system. | |
| − | + | * Perfect cleavage in three directions (60 and 120 degree angles). | |
| − | + | * Streak = scarlet. | |
| − | + | * Fracture = subconchoidal to uneven. | |
| − | + | * Luster = adamantine to dull. | |
| − | + | * Ground cinnabar has uneven sized fractured particles and may contain quartz. | |
| − | = | + | * Transmitted light color is cherry red. |
| + | * Mohs Hardness = 2.0 - 2.5 | ||
| + | * Density = 8.10-8.176 g/ml | ||
| + | * Refractive Index = 3.146; 2.819 | ||
| + | ==Resources and Citations== | ||
| + | * Ruth Siddall, 'Mineral Pigments in Archaeology: Their Analysis and the Range of Available Materials' ''Minerals'' Vol 8, p. 201 (2018). [https://www.academia.edu/36588315/Mineral_Pigments_in_Archaeology_Their_Analysis_and_the_Range_of_Available_Materials?email_work_card=view-paper Link] | ||
| + | * R.Gettens, R.Feller, W.Chase, "Vermilion and Cinnabar", ''Artists Pigments'', Volume 2, A. Roy (ed.), Oxford University Press: Oxford, 1993. ° Mineralogy Database: [http://www.webmineral.com/data/Cinnabar.shtml Cinnabar] | ||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density = 8.1; ref. index = 3.146; 2.819 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density = 8.1; ref. index = 3.146; 2.819 | ||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 200 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 200 | ||
| − | |||
* Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | ||
| − | |||
* ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | * ''Dictionary of Building Preservation'', Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996 | ||
| − | |||
* Henry Hodges, ''Artifacts: An Introduction to Early Materials and Technology'', Ronald P. Frye, Kingston, Canada, 1988 | * Henry Hodges, ''Artifacts: An Introduction to Early Materials and Technology'', Ronald P. Frye, Kingston, Canada, 1988 | ||
| − | |||
* C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | * C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | ||
| − | |||
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=8.12 | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=8.12 | ||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| − | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Cinnabar." Accessed 12 May 2004. | |
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "Cinnabar." | + | * Wikipedia: http://en.wikipedia.org/wiki/Cinnabar (Accessed Sept. 7, 2005) |
| − | |||
| − | * Wikipedia | ||
| − | |||
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | |||
* Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
| − | |||
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| − | |||
* Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | ||
Latest revision as of 10:52, 1 March 2024
Description
A soft, dense, red, native ore composed of mercuric sulfide. Cinnabar is widely distributed around the world and is most often found in veins near volcanic rocks or hot springs. It has been mined from the Spanish cliffs near Almadén for over 2000 years. Other deposits are found in Italy (Iudrio), Germany, Austria, Yugoslavia, China (Hunan, Kweichow), Turkestan, Mexico, Peru (Huancavelica) and the United States (Texas, California, Nevada). When finely ground cinnabar produces a bright red opaque pigment that has been used since antiquity. Red mercuric sulfide has been made made synthetically since at least the 8th century in Europe and possibly earlier in China. Synthetic mercuric sulfide is called Vermilion. Due to impurities, vermilion was favored over ground cinnabar as a red pigment. When exposed to ultraviolet light, mercuric sulfide darkens as a portion changes from its normal red crystalline form to a black isomorph. This can result in splotchy discolorations.
Synonyms and Related Terms
red mercuric sulfide; Pigment Red 106; CI 77766; Zinnober (Deut.); Zinnoberersatz (Ned.); cinabrio (Esp.); cinabre (Fr.); cinabro (It.); cinábrio (Port.); cinnaber (Ned.); shinsha (Jap.); tan-sha (Chin.); sulfure de mercure (Fr.); Chinese vermilion; English vermilion; vermilion (synthetic pigment); red sulfuretted oxide of mercury; liver ore
Risks
- Highly toxic by ingestion, inhalation and skin absorption.
- Darkens in ultraviolet light.
- Kremer-Pigmente: MSDS
Physical and Chemical Properties
- Composition = HgS
- Soluble in aqua regia. Unaffected by alkalis.
- Hexagonal crystal system.
- Perfect cleavage in three directions (60 and 120 degree angles).
- Streak = scarlet.
- Fracture = subconchoidal to uneven.
- Luster = adamantine to dull.
- Ground cinnabar has uneven sized fractured particles and may contain quartz.
- Transmitted light color is cherry red.
- Mohs Hardness = 2.0 - 2.5
- Density = 8.10-8.176 g/ml
- Refractive Index = 3.146; 2.819
Resources and Citations
- Ruth Siddall, 'Mineral Pigments in Archaeology: Their Analysis and the Range of Available Materials' Minerals Vol 8, p. 201 (2018). Link
- R.Gettens, R.Feller, W.Chase, "Vermilion and Cinnabar", Artists Pigments, Volume 2, A. Roy (ed.), Oxford University Press: Oxford, 1993. ° Mineralogy Database: Cinnabar
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density = 8.1; ref. index = 3.146; 2.819
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 200
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Henry Hodges, Artifacts: An Introduction to Early Materials and Technology, Ronald P. Frye, Kingston, Canada, 1988
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=8.12
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Encyclopedia Britannica, http://www.britannica.com Comment: "Cinnabar." Accessed 12 May 2004.
- Wikipedia: http://en.wikipedia.org/wiki/Cinnabar (Accessed Sept. 7, 2005)
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985