Difference between revisions of "Copper-8-quinolate"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 9: | Line 9: | ||
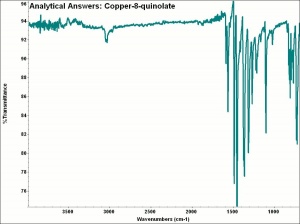
[[[SliderGallery rightalign|aaiCOPPER8.jpg~FTIR]]] | [[[SliderGallery rightalign|aaiCOPPER8.jpg~FTIR]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Toxic by ingestion. | ||
| + | * Gelest: ]https://www.gelest.com/wp-content/uploads/AKC253.2_COPPER-8-HYDROXYQUINOLINATE_GHS-US_English-US.pdf SDS] | ||
| + | ==Physical and Chemical Properties== | ||
Soluble in strong acids. Slightly soluble in weak acids, pyridine, quinoline. Insoluble in water and most organic solvents. | Soluble in strong acids. Slightly soluble in weak acids, pyridine, quinoline. Insoluble in water and most organic solvents. | ||
| Line 22: | Line 26: | ||
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.63 | + | | 1.63 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 28: | Line 32: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
Latest revision as of 15:44, 4 July 2022
Description
Yellow-green, nonhygroscopic, odorless powder. Copper-8-quinolate is used as a fungicide. It is often used in fish and animal glues.
Synonyms and Related Terms
copper-8; copper oxinate; oxine copper; copper-8-hydroxyquinoline; Cunilate 2174-NO
Risks
- Toxic by ingestion.
- Gelest: ]https://www.gelest.com/wp-content/uploads/AKC253.2_COPPER-8-HYDROXYQUINOLINATE_GHS-US_English-US.pdf SDS]
Physical and Chemical Properties
Soluble in strong acids. Slightly soluble in weak acids, pyridine, quinoline. Insoluble in water and most organic solvents.
| Composition | Cu(C9H6ON)2 |
|---|---|
| CAS | 10380-28-6 |
| Density | 1.63 g/ml |
| Molecular Weight | mol. wt. = 351.9 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Conservation Materials Ltd., Catalog