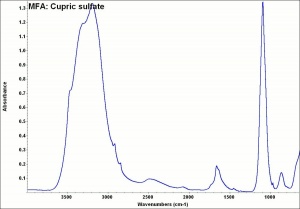
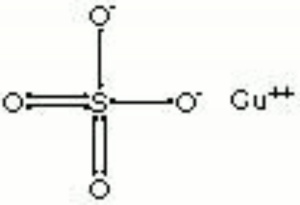
Copper sulfate
Description
Blue granules or triclinic crystals. Copper sulfate occurs in nature as the minerals Chalcanthite (hydrated) and hydrocyanite (anhydrous). It is made synthetically by the reaction of Sulfuric acid with Copper or copper oxide. Copper sulfate (chalcanthite) is also found as a corrosion product on outdoor copper and copper alloys in areas with sulfur dioxide pollution. Copper sulfate was a major source of copper or in early civilizations. It has also been used as a mordant and as a bluish green dye. Copper sulfate is used as a biocide and as a preservative for Wood, Paper and Leather. It is also used in indelible inks, in metal coloring baths and as a copper print toner in photography. Anhydrous copper sulfate quickly absorbs water, turning blue. As a chemical reagent, blue copper sulfate reacts with reducing sugars to form red copper oxide.
See also dibasic copper sulfate (Antlerite) and tribasic copper sulfate (Brochantite)
Synonyms and Related Terms
copper sulfate pentahydrate; copper sulphate (Br.); cupric sulfate; blue vitriol; bluestone; blue copperas; vitriol of Venus; Roman vitriol; Salzburg vitriol; Kupfersulfat (Deut.); Kupfervitriol (Deut.); sulfate de cuivre (Fr.); kopersulfaat (Ned.); siarczan miedzi (Pol.); sulfato de cobre (Port.)
Other Properties
Soluble in water, methanol. Slightly soluble in ethanol, glycerol.
| Composition | CuSO4-5H2O |
|---|---|
| CAS | 7758-98-7 |
| Melting Point | 150 |
| Density | 2.284 |
| Molecular Weight | mol. wt. = 159.60 |
| Refractive Index | 1.724, 1.733, 1.739 |
Hazards and Safety
Toxic by ingestion. Strongly irritating to skin and membranes.
Mallinckrodt Baker: MSDS
Sources Checked for Data in Record
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry #2722
- Palmy Weigle, Ancient Dyes for Modern Weavers, Watson-Guptill Publications, New York, 1974
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- John and Margaret Cannon, Dye Plants and Dyeing, Herbert Press, London, 1994
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Copper_sulfate
- Website address 1 Comment: conservation termlist - www.hants.org.uk/museums
- Website address 2 Comment: photographic chemicals - www.jetcity.com/~mrjones/chemdesc.htm
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.724, 1.733, 1.739