Difference between revisions of "Diphenylcarbazone"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 2: | Line 2: | ||
Orange-red needles that are used as a colorimetric reagent for the detection of soluble [[mercury]] salts in ethnographic and natural science collections (Odegaard et al 2000). Diphenylcarbazone dissolves in [[ethyl alcohol|ethanol]] and turns blue when mercury is present. | Orange-red needles that are used as a colorimetric reagent for the detection of soluble [[mercury]] salts in ethnographic and natural science collections (Odegaard et al 2000). Diphenylcarbazone dissolves in [[ethyl alcohol|ethanol]] and turns blue when mercury is present. | ||
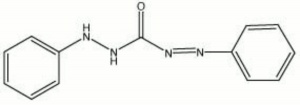
| − | + | [[[SliderGallery rightalign|diphenylcarbazone.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
phenyldiazenecarboxylic acid 2-phenylhydrazide; phenylazoformic acid 2-phenylhydrazide | phenyldiazenecarboxylic acid 2-phenylhydrazide; phenylazoformic acid 2-phenylhydrazide | ||
| − | + | == Risks == | |
| − | |||
| − | == | ||
| + | * May be harmful if ingested or inhaled. | ||
| + | * Contact may cause irritation. | ||
| + | * Millipore Sigma: [https://www.sigmaaldrich.com/US/en/product/vetec/v000588 SDS] | ||
| + | ==Physical and Chemical Properties== | ||
Insoluble in water. Soluble in alcohol, chloroform, benzene. | Insoluble in water. Soluble in alcohol, chloroform, benzene. | ||
| Line 22: | Line 24: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 157 | + | | 157 C |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 28: | Line 30: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 3334 | * ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 3334 | ||
| − | * N.Odegaard, S.Carroll, W.Zimmt, ''Material Characterization Tests for Objects of Art and Archaeology'', Archetype Publications, London, 2000 | + | * N.Odegaard, S.Carroll, W.Zimmt, ''Material Characterization Tests for Objects of Art and Archaeology'', Archetype Publications, London, 2000, p. 72. |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 17:08, 21 July 2022
Description
Orange-red needles that are used as a colorimetric reagent for the detection of soluble Mercury salts in ethnographic and natural science collections (Odegaard et al 2000). Diphenylcarbazone dissolves in ethanol and turns blue when mercury is present.
Synonyms and Related Terms
phenyldiazenecarboxylic acid 2-phenylhydrazide; phenylazoformic acid 2-phenylhydrazide
Risks
- May be harmful if ingested or inhaled.
- Contact may cause irritation.
- Millipore Sigma: SDS
Physical and Chemical Properties
Insoluble in water. Soluble in alcohol, chloroform, benzene.
| Composition | C13H12N4O |
|---|---|
| CAS | 538-62-5 |
| Melting Point | 157 C |
| Molecular Weight | mol. wt. = 240.26 |
Resources and Citations
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 3334
- N.Odegaard, S.Carroll, W.Zimmt, Material Characterization Tests for Objects of Art and Archaeology, Archetype Publications, London, 2000, p. 72.