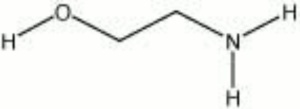
Ethanolamine
Description
A colorless, viscous liquid with an ammonia-like odor that is widely used commercially. Ethanolamines are used in nonionic detergents and as solvents in dry cleaning. They are hygroscopic and are used as humectants to soften hides and to condition wool. Ethanolamines are used as corrosion inhibitors because they are an effective scavenger for sulfur containing gases. The soaps of ethanolamines are used in shampoos, as surfactants and as emulsifiers.
See also diethanolamine, and triethanolamine.
Synonyms and Related Terms
MEA; monoethanolamine; colamine; 2-aminoethanol; 2-hydroxyethylamine; ethylolamine; beta-aminoethyl alcohol; M-251; ethanolamine; beta-aminoethanol; glycinol
Other Properties
Miscible in water, alcohol, methanol, acetone, chloroform.
| Composition | HOCH2CH2NO2 |
|---|---|
| CAS | 141-43-5 |
| Melting Point | 10.3 |
| Density | 1.0117 |
| Molecular Weight | mol. wt.=61.08 |
| Boiling Point | 170.8+ |
Hazards and Safety
Combustible. Flash point = 85C (185 F).
Mildly toxic by ingestion. Corrosive. Inhalation and skin contact cause severe irritation.
Mallinckrodt Baker: MSDS
Authority
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry # 3772