Difference between revisions of "Ultramarine violet"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 9: | Line 9: | ||
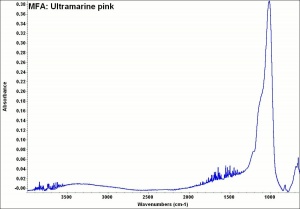
[[[SliderGallery rightalign|MFA- Ultramarine pink.jpg~FTIR]]] | [[[SliderGallery rightalign|MFA- Ultramarine pink.jpg~FTIR]]] | ||
| − | == | + | == Risks == |
| − | + | * No significant hazards. | |
| + | * Noncombustible. | ||
| − | + | ==Physical and Chemical Properties== | |
| − | ASTM lightfastness=1 (excellent). | + | * Discolors when exposed to weak acids or sulfur fumes. |
| + | * Insoluble in water. | ||
| + | * Fine, transparent violet grains mixed with deep blue grains. | ||
| + | * Isotropic. | ||
| + | * ASTM lightfastness=1 (excellent). | ||
| + | * Refractive Index = 1.56 | ||
| − | + | ==Resources and Citations== | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * J. Plesters, "Ultramarine Blue, Natural and Artificial", ''Artists Pigments'', Volume 2, A. Roy (ed.), Oxford University Press: Oxford, 1993. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | J. Plesters, "Ultramarine Blue, Natural and Artificial", ''Artists Pigments'', Volume 2, A. Roy (ed.), Oxford University Press: Oxford, 1993. | ||
| − | |||
| − | |||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
Revision as of 10:00, 23 June 2022
Description
A rosy violet pigment that is a color variation produced in the manufacture of synthetic ultramarine blue. Ultramarine violets and reds were developed in Germany about 1875. The pigments are produced by treating ultramarine blue with sal ammoniac or dry hydrochloric acid gas at high temperatures. The sodium reacts to form sodium chloride, which is then washed away. Ultramarine violet is a stable pigment but it has poor tinting strength and is rarely used in artists' paints (Plesters, 1993).
Synonyms and Related Terms
Pigment Violet 15; mineral violet; violet outremer (Fr.); Ultramarinviolett (Deut.); violetto oltremare (It.); ultramarijn violet (Ned.); violeta de ultramarino (Port.); ultramarine red; ultramarine pink;
Risks
- No significant hazards.
- Noncombustible.
Physical and Chemical Properties
- Discolors when exposed to weak acids or sulfur fumes.
- Insoluble in water.
- Fine, transparent violet grains mixed with deep blue grains.
- Isotropic.
- ASTM lightfastness=1 (excellent).
- Refractive Index = 1.56
Resources and Citations
- J. Plesters, "Ultramarine Blue, Natural and Artificial", Artists Pigments, Volume 2, A. Roy (ed.), Oxford University Press: Oxford, 1993.
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments"