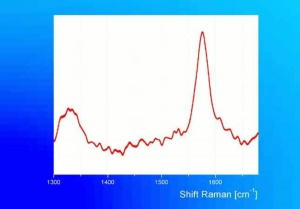
Graphite
Description
A naturally occurring silvery-black allotropic form of Carbon. Graphite is formed by horizontal sheets of hexagonal carbon rings. The layered structure makes makes it soft, slippery, and flaky. A graphite deposit was discovered in England and mined for two centuries to produce small lumps of wadd, or black lead, that were used for writing. By the 18th century, graphite was mixed with Clay to form pencil lead. Current graphite mines are found in Sri Lanka, Madagascar, India, North Korea, Mexico (Sonora), Ontario, western Siberia, and the U.S. (New York, New Jersey, Alabama, Texas). Graphite is extremely stable at high temperatures and does not conduct heat. It is used as crucibles, metal molds, brick, electrodes, lubricants, stove polish, and as a pigment for industrial paints. It is not used as an artist pigment. Graphite was first made synthetically by Edward G. Acheson (patented 1896). Nearly pure graphite is manufactured from anthracite coal and petroleum coke.
Synonyms and Related Terms
Pigment Black 10; CI 77265; grafit (Dan., Pol., Sven.); Graphit (Deut.); grafito (Esp.); graphite (Fr.); grafitis (Gr.); grafite (It., Port.); grafiet (Ned.); black lead; plumbago; wadd; hypercarburet of iron; Flanders stone; stove black;
Other Properties
Cleavage = perfect in one direction Crystals = hexagonal tablets, thin flakes.
Luster = metallic Streak = gray to black
| Composition | C |
|---|---|
| CAS | 7782-42-5 |
| Mohs Hardness | 1.0 - 2.0 |
| Density | 2.0-2.36 |
| Refractive Index | opaque |
Hazards and Safety
The finely powdered dust is toxic by inhalation. Fire risk.
Mallinckrodt Baker: MSDS
Additional Information
° J.Winter, "The Characterization of Pigments Based on Carbon" Studies in Conservation, 28:49-66, 1983.
° Mineralogy Database: Graphite
Additional Images
Authority
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density=2.36; ref. ind=opaque
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Encyclopedia Britannica, http://www.britannica.com Comment: "graphite" Encyclopædia Britannica [Accessed December 11, 2001;
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=2.30-2.72