Difference between revisions of "Gypsum"
m (→Additional Images: added captions for last 2 images) |
|||
| Line 10: | Line 10: | ||
native calcium sulfate; alabaster; selenite; terra alba; satinite; mineral white; satin spar; light spar; sulfate of lime; puritan filler; crown filler; Pigment White 25; Gips (Deut.); gips (Ned., Pol.); yeso (Esp.); gypse (Fr.); gesso (It., Port.) | native calcium sulfate; alabaster; selenite; terra alba; satinite; mineral white; satin spar; light spar; sulfate of lime; puritan filler; crown filler; Pigment White 25; Gips (Deut.); gips (Ned., Pol.); yeso (Esp.); gypse (Fr.); gesso (It., Port.) | ||
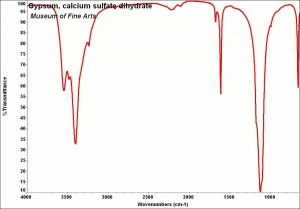
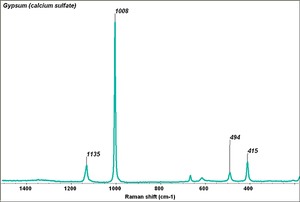
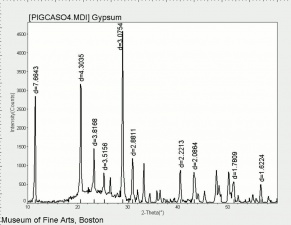
| − | [[[SliderGallery rightalign| | + | [[[SliderGallery rightalign|Gypsum, calcium sulfate dihydrate.jpg~FTIR|Gypsum (calcium sulfate) copy.tif~Raman (MFA)|PIGCASO4.jpg~XRD]]] |
== Other Properties == | == Other Properties == | ||
Revision as of 11:12, 14 November 2019
Description
A soft, transparent, easily cleaved mineral composed of hydrated calcium sulfate. Gypsum is found as twinned monoclinic crystals, called Selenite, or silky fibrous crystals, called Satin spar. Massive blocks of fine-grain white, translucent gypsum are called Alabaster and have been used since ancient times for carved ornamental objects and statuary. Gypsum is a commonly found mineral associated with sedimentary rock and deposits from seas, lakes, and volcanic springs (Gypcrete). For a long time, gypsum quarries in the Montmartre district of Paris supplied the starting material for the burnt gypsum that was, and still is, called Plaster of Paris. Raw gypsum is used for carvings (Alabaster), for wallboards (Sheetrock®), as a filler in paper (Crown filler), as a paint pigment (Terra alba) and as an ingredient in Portland cement. Finely ground gypsum was mixed with rabbit skin glue and used as Gesso.
Synonyms and Related Terms
native calcium sulfate; alabaster; selenite; terra alba; satinite; mineral white; satin spar; light spar; sulfate of lime; puritan filler; crown filler; Pigment White 25; Gips (Deut.); gips (Ned., Pol.); yeso (Esp.); gypse (Fr.); gesso (It., Port.)
Other Properties
Soluble in water. Slightly soluble in glycerol and weak acids. Precipitates as needle-like crystals. Insoluble in most organic solvents. Gypsum fluoresces purple.
Microscopic identification = in plane polarized light (PPL), colorless with moderate relief. Perfect cleavage in one direction and good cleavage in two directions. Some samples may contain well-formed rhombic or columnar particles. Some particles exhibit twinning with 'swallowtail' shapes. RI < 1.662. In cross-polarized light (XPL), low birefringence with first-order grays observed. Extinction is inclined.
Luster = vitreous, silky or pearly. Streak = white. Fracture = conchoidal to splintery. Euhedral shaped crystals contain numerous inclusions.
| Composition | CaSO4-2H2O |
|---|---|
| CAS | 1010-14-4 |
| Mohs Hardness | 1.5 - 2.0 |
| Melting Point | 100-150 |
| Density | 2.32-2.36 |
| Molecular Weight | mol. wt. = 172.2 |
| Refractive Index | 1.520; 1.523; 1.530 |
Hazards and Safety
Inhalation and contact may cause slight allergies.
LINK: International Chemical Safety Card
Additional Information
Mineralogy Database: Gypsum
Comparisons
Characteristics of Common White Pigments
Additional Images
Sources Checked for Data in Record
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: Refractive Index: alpha=1.519-1.521; beta=1.522-1.523; gamma=1.529-1.530
- Encyclopedia Britannica, http://www.britannica.com Comment: "gypsum" [Accessed December 4, 2001 (B/W photo)
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Gypsum (Accessed Nov. 2, 2005)
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density = 2.36 and ref.index =.1.520 ;1.530; 1.523
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 385
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Sue Fuller, Rocks and Minerals, DK Publishing, Inc., New York City, 1995
- Jack Odgen, Jewellery of the Ancient World, Rizzoli International Publications Inc., New York City, 1982
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980 Comment: ref. index = 1.21; 1.52; 1.53
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1753
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=2.31-2.33