Difference between pages "Sodium metasilicate" and "Sodium nitrate"
(Difference between pages)
Jump to navigation
Jump to search
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | + | Colorless, deliquescent crystals that naturally as [[caliche|caliche]] in mineral deposits. Sodium nitrate is primarily used as a fertilizer. It is also used in the manufacture of glass, match heads, and explosives. | |
| − | == | + | == Synonyms and Related Terms == |
| − | + | caliche; Chili niter; Chile nitre; Chile saltpeter; soda niter; cubic niter; dusiènan sodný (Ces.); Natriumnitrat (Deut.); nitrato sódico (Esp.); nitrate de sodium (Fr.); natriumnitraat (Ned.); azotan(V) sodu (Pol.); | |
| − | * | + | |
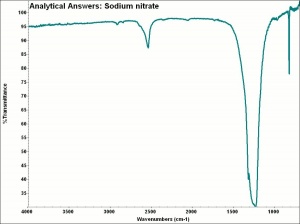
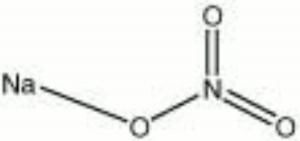
| − | * | + | [[[SliderGallery rightalign|aaiNANO3.jpg~FTIR|sodium nitrate.jpg~Chemical structure]]] |
| − | * Contact | + | |
| − | * | + | == Hazards and Safety == |
| + | |||
| + | * Toxic by ingestion and inhalation. | ||
| + | * Hygroscopic. | ||
| + | * Contact may cause irritation. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=S343500&productDescription=SOD+NITRATE+ACS+500G&vendorId=VN00033897&countryCode=US&language=en SDS] | ||
== Physical and Chemical Properties == | == Physical and Chemical Properties == | ||
| − | Soluble in water | + | Soluble in water, glycerol. Slightly soluble in ethanol. Crystals are cubic. |
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
! scope="row"| Composition | ! scope="row"| Composition | ||
| − | | | + | | NaNO3 |
|- | |- | ||
! scope="row"| CAS | ! scope="row"| CAS | ||
| − | | | + | | 7631-99-4 |
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | | + | | 308 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2. | + | | 2.267 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| − | | mol. wt. = | + | | mol. wt. = 85 |
| + | |- | ||
| + | ! scope="row"| Refractive Index | ||
| + | | 1.5874, 1.3361 | ||
| + | |- | ||
| + | ! scope="row"| Boiling Point | ||
| + | | 380 C (dec) | ||
|} | |} | ||
| + | |||
| + | == Physical and Chemical Properties == | ||
| + | |||
| + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 738 | ||
| + | |||
| + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| + | |||
| + | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | ||
| + | |||
| + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 | ||
| + | |||
| + | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| + | |||
| + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
| + | |||
| + | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8792 | ||
| + | |||
| + | * Wikipedia: http://en.wikipedia.org/wiki/Sodium_nitrate (Accessed Jan. 15, 2006) | ||
| + | |||
| + | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index = 1.5874, 1.3361 | ||
| + | |||
| + | |||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 09:25, 2 June 2022
Description
Colorless, deliquescent crystals that naturally as Caliche in mineral deposits. Sodium nitrate is primarily used as a fertilizer. It is also used in the manufacture of glass, match heads, and explosives.
Synonyms and Related Terms
caliche; Chili niter; Chile nitre; Chile saltpeter; soda niter; cubic niter; dusiènan sodný (Ces.); Natriumnitrat (Deut.); nitrato sódico (Esp.); nitrate de sodium (Fr.); natriumnitraat (Ned.); azotan(V) sodu (Pol.);
Hazards and Safety
- Toxic by ingestion and inhalation.
- Hygroscopic.
- Contact may cause irritation.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water, glycerol. Slightly soluble in ethanol. Crystals are cubic.
| Composition | NaNO3 |
|---|---|
| CAS | 7631-99-4 |
| Melting Point | 308 C |
| Density | 2.267 g/ml |
| Molecular Weight | mol. wt. = 85 |
| Refractive Index | 1.5874, 1.3361 |
| Boiling Point | 380 C (dec) |
Physical and Chemical Properties
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 738
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8792
- Wikipedia: http://en.wikipedia.org/wiki/Sodium_nitrate (Accessed Jan. 15, 2006)
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index = 1.5874, 1.3361