Difference between revisions of "Hydroxyacetic acid"
Jump to navigation
Jump to search
m (Text replace - "== Authority ==" to "== Sources Checked for Data in Record ==") |
|||
| Line 9: | Line 9: | ||
[[[SliderGallery rightalign|hydroxyacetic acid.jpg~Chemical structure]]] | [[[SliderGallery rightalign|hydroxyacetic acid.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | Corrosive. Contact causes irritation and burns. | ||
| + | |||
| + | ThermoFisher: [https://www.fishersci.com/msdsproxy%3FproductName%3DO610850%26productDescription%3DGLYCOLIC%2BACID%2BCERT%2B50G%26catNo%3DO6108-50%26vendorId%3DVN00033897%26storeId%3D10652 SDS] | ||
| + | == Physical and Chemical Properties == | ||
Soluble in water, methanol, ethanol, acetone, acetic acid, ether. | Soluble in water, methanol, ethanol, acetone, acetic acid, ether. | ||
| Line 31: | Line 36: | ||
|} | |} | ||
| − | == | + | == Resources and Citations == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 | ||
Revision as of 10:41, 19 August 2020
Description
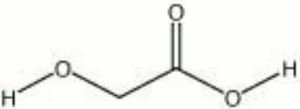
Colorless, deliquescent crystals that occur naturally as a component in sugarcane. Hydroxyacetic acid, or glycolic acid, is a weak acid. It is sold commercially as a 70% solution. It is used in processing and dyeing textiles and Leather. Hydroxyacetic acid is also used for cleaning, polishing, and soldering metals.
Synonyms and Related Terms
glycolic acid; hydroxyethanoic acid
Risks
Corrosive. Contact causes irritation and burns.
ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water, methanol, ethanol, acetone, acetic acid, ether.
| Composition | CH2OHCOOH |
|---|---|
| CAS | 79-14-1 |
| Melting Point | 80 |
| Density | 1.27 |
| Molecular Weight | mol. wt. = 76.05 |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 4508