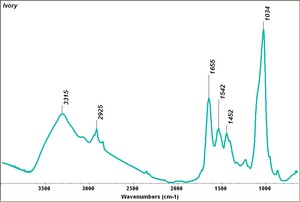
Ivory
Description
Long, curved elephant tusks that are rootless, non-enameled incisors. The size and structure of a tusks depends on the animal, its age and its living conditions. Tusks as long as 6-8 feet have been obtained from African elephants. The Indian elephants produce tusks about 4-5 feet long. Ivory is a relatively soft, workable but durable material that is white to pale yellow in color. Elephant tusks are primarily composed of dentin, a hard calcareous material composed of Calcium hydroxyapatite and protein with small amounts of Calcium carbonate, Calcium fluoride, and Magnesium phosphate. A new layer of dentin is added each season. This produces a layered ring structure that can be seen in fresh ivory. Deteriorated ivory tends to flake and peel along these lines. Ivory was widely traded from prehistoric times. It was considered valuable by all cultures and widely used in utilitarian objects, jewelry, sculpture, seals, game-pieces, furniture, marquetry and scientific instruments. Ivory reached its peak periods of use in the 13th and 14th centuries. From 1976 to 1989, more than 100 nations banned ivory imports from the Asian elephant; African elephant ivory was added to the ban in 1989. Mammoth ivory is not banned.
The CITES Identification Guide for Ivory and Ivory Substitutes (2020) is available at: link
Synonyms and Related Terms
elephant tusk; dentine; dentin; ivoire (Fr.); marfil (Esp.); marfim (Port.); Elfenbein (Deut.); ivoor (Ned.)
Physical and Chemical Properties
- Ivory is dense with compact ores
- Elephant ivory and mammoth ivory can be differentiated microscopically by their Schreger patterns of intersecting arcs (Espinoza and Mann 1993).
- UV autofluorescence ranges from white to purple or blue for elephant ivory and white to yellow for mammoth ivory.
- Mohs Hardness = 2-3
- Density = 1.70-1.93
- Refractive Index = 1.539, 1.541
Additional Images
Resources and Citations
- J.Wornoff, "Identification of Ivory Covers and Comparison to Ivory Portrait Miniatures" e-conservation magazine No. 16:24-38, 2010 at link
- F.Minney, "Ivory" The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996.
- E.Espinoza, J-J.Mann "The History and Significance of the Schreger Pattern in Proboscidean Ivory Characterization" JAIC, 32:241-248, 1993.
- C.Snow, T.Weisser, "The Examination and Treatment of Ivory and Related Materials", IIC Conference, Adhesives and Consolidants, 1984.
- J.Thornton,"The Structure of Ivory and Ivory Substitutes", AIC Preprints, Philadelphia, 1981, p.173-181.
- Encyclopedia Britannica, http://www.britannica.com Comment: ivory" [Accessed October 18, 2001].
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Oppi Untracht, Jewelry Concepts and Technology, Doubleday & Co., Inc., New York City, 1985
- Benjamin Burack, Ivory and Its Uses, Charles E. Tuttle and Co., Vermont, 1984
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=1.83-1.92; ref. index=1.539, 1.541