Difference between revisions of "Litharge"
(username removed) |
(username removed) |
||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | A heavy yellow powder composed of [http://cameo.mfa.org/materials/fullrecord.asp?name=lead | + | A heavy yellow powder composed of [http://cameo.mfa.org/materials/fullrecord.asp?name=lead%20monoxide lead monoxide]. Litharge is prepared as the oxidized product of molten lead that has been stirred or atomized to incorporate air then cooled and ground to form the yellow powder. [http://cameo.mfa.org/materials/fullrecord.asp?name=Massicot Massicot], another crystalline form of lead monoxide, occurs naturally but can also be made by heating [http://cameo.mfa.org/materials/fullrecord.asp?name=lead%20carbonate%2C%20basic lead carbonate] to 300C. Litharge is lightly more orange than massicot due to some formation of [http://cameo.mfa.org/materials/fullrecord.asp?name=red%20lead%20 red lead oxide]. Both forms of lead monoxide has been used as a [http://cameo.mfa.org/materials/fullrecord.asp?name=drier drier] in [http://cameo.mfa.org/materials/fullrecord.asp?name=oil oil] and as a low-fire [http://cameo.mfa.org/materials/fullrecord.asp?name=flux flux] in making [http://cameo.mfa.org/materials/fullrecord.asp?name=ceramic ceramics] and [http://cameo.mfa.org/materials/fullrecord.asp?name=glass glass]. They were used as a yellow pigments in [http://cameo.mfa.org/materials/fullrecord.asp?name=paint paints] and [http://cameo.mfa.org/materials/fullrecord.asp?name=glaze glazes]. Thin layers of lead monoxide are used to produce iridescent colors on [http://cameo.mfa.org/materials/fullrecord.asp?name=brass brass] and [http://cameo.mfa.org/materials/fullrecord.asp?name=bronze bronze]. It has also been used as a filler for [http://cameo.mfa.org/materials/fullrecord.asp?name=rubber%2C%20natural rubber] and to produce artificial [http://cameo.mfa.org/materials/fullrecord.asp?name=tortoiseshell tortoiseshell] and [http://cameo.mfa.org/materials/fullrecord.asp?name=horn horn]. Litharge is mixed with [http://cameo.mfa.org/materials/fullrecord.asp?name=glycerol glycerol] to make [http://cameo.mfa.org/materials/fullrecord.asp?name=litharge%20cement plumber's cement]. |
[[File:litharge2 C100x.jpg|thumb|litharge]] | [[File:litharge2 C100x.jpg|thumb|litharge]] | ||
Revision as of 12:03, 13 June 2013
Description
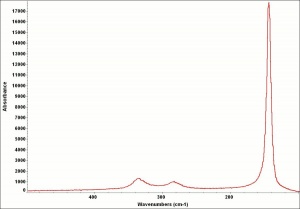
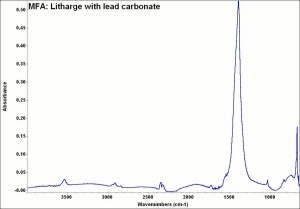
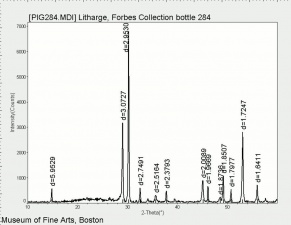
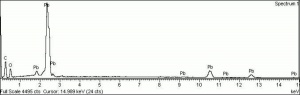
A heavy yellow powder composed of lead monoxide. Litharge is prepared as the oxidized product of molten lead that has been stirred or atomized to incorporate air then cooled and ground to form the yellow powder. Massicot, another crystalline form of lead monoxide, occurs naturally but can also be made by heating lead carbonate to 300C. Litharge is lightly more orange than massicot due to some formation of red lead oxide. Both forms of lead monoxide has been used as a drier in oil and as a low-fire flux in making ceramics and glass. They were used as a yellow pigments in paints and glazes. Thin layers of lead monoxide are used to produce iridescent colors on brass and bronze. It has also been used as a filler for rubber and to produce artificial tortoiseshell and horn. Litharge is mixed with glycerol to make plumber's cement.
Synonyms and Related Terms
massicot; lead monoxide; lithargyros (Gr.); plumbum ustum (Latin); Lithargit (Deut.); Massicot (Deut.); litharge (Fr.); litargirio (Esp.); litargirio (giallo di piombo) (It.); massicot (Ned.); litharge (Ned.); litargrio (Port.); yellow lead oxide; plumbous oxide
Other Properties
Soluble in acetic acid, dilute nitric acid and alkalis. Insoluble in water and ethanol. Turns gray on exposure to sulfur fumes.
| Composition | PbO |
|---|---|
| CAS | 1317-36-8 |
| Melting Point | 888 |
| Density | 9.40-9.53 |
| Refractive Index | 2.51; 2.71; 2.61 |
Hazards and Safety
Toxic by inhalation or ingestion. Skin contact may cause irritation or ulcers. Carcinogen, teratogen, suspected mutagen.
LINK: International Chemical Safety Card
Authority
- R. J. Gettens, G.L. Stout, R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density 9.40 and ref. index 2.51; 2.71; 2.61
- Thomas Gregory, Thomas Gregory, The Condensed Chemical Dictionary, Reinhold Publishing, New York, 3rd ed., 1942
- Ralph Mayer, Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Susan E. Schur, Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996