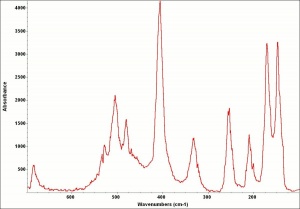
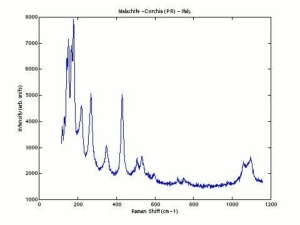
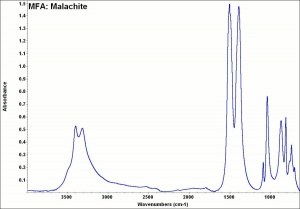
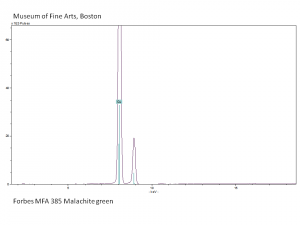
Malachite
Description
A green mineral composed of Basic copper carbonate. Malachite occurs naturally with the blue copper carbonate mineral called Azurite with malachite being the more abundant of the two. Major deposits of the copper ores have been found in Siberia (Nizhne-Tagilsk), France (Chessy), Nambia (Tsumeb), Congo, Australia (New South Wales), and the U.S. (Bisbee, Arizona). Both malachite and azurite have been used as gemstones and paint pigments since before 3000 BCE. Malachite is prepared as pigment by careful selection, grinding, washing, and levigation. Coarsely ground malachite gives a dark green color while finely ground particles give a lighter more transparent tone. Malachite is a lightfast but is sensitive to acids and sulfur fumes. Basic copper carbonate can also be made artificially by coloring chalk with Copper sulfate. The synthetic pigment, called Green verditer, tends to have regularly sized particles with rounded edges with a paler color than malachite. Natural malachite was occasionally used in tempera based paintings before the 16th century and later, green verditer was used for both distemper and oil based interior house paints in the 19th century. Malachite is also found as a corrosion product on metallic Copper.
Synonyms and Related Terms
basic copper carbonate; Pigment Green 39; CI 77492; Berggrün (Deut.); Malachit (Deut.); verdetto della Magna (It.); malachito (It.) malaquita (Esp.); malaquite (Port.); malachite (Fr., It.); malachitis (Gr.); malachiet (Ned.); rokusho (Jap.); iwarokusho (Jap.); byaku roku (Jap.); shih lü; (Chin.); spang (Tibetan); verde azzurro; basic cupric carbonate; green hydrous copper carbonate; green bice; Bremen green; green verditer; Hungarian green; mountain green; mineral green; copper green; iris green; Olympian green
Other Properties
Insoluble in water and ethanol. Decomposes in acids with the evolution of carbon dioxide bubbles. Decomposes with warm alkalis. Stable in light.
Perfect cleavage in one direction. Streak = green. It is a brittle mineral that fractures conchoidally with splinters. Pigment particles are coarse and irregular.
| Composition | CuCO3-Cu(OH)2 |
|---|---|
| CAS | 12069-69-1 |
| Mohs Hardness | 3.5 - 4.0 |
| Density | 3.9-4.0 |
| Refractive Index | 1.655; 1.909; 1.875 |
Hazards and Safety
Skin contact and inhalation may cause irritation or allergic reactions. Chronic exposure may cause anemia.
Turns black with warm alkalis, hydrogen sulfide or sulfur fumes.
Additional Information
° R. Gettens, and E. West FitzHugh, "Malachite and Green Verditer", Artists Pigments, Vol. 2., A. Roy ed., Oxford University Press, Oxford, 1993. ° Mineralogy Database: Malachite
Comparisons
Additional Images
- Image3 802333.jpg
Side view of malachite
Authority
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- Artists' Pigments: A Handbook of their History and Characteristics, Ashok Roy (ed.), National Gallery of Art, Washington DC, Vol. 2, 1993 Comment: R. Gettens, and E. West FitzHugh, "Malachite and Green Verditer"
- Encyclopedia Britannica, http://www.britannica.com Comment: Retrieved June 5, 2003, from Encyclopædia Britannica Premium Service.
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: 'Pigments'
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Malachite (Accessed Sept. 10, 2005)
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density=4.0, ref index=1.655; 1.909; 1.875
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- C.V.Horie, Materials for Conservation, Butterworth-Heineman, London, 1997
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Jack Odgen, Jewellery of the Ancient World, Rizzoli International Publications Inc., New York City, 1982
- A.Lucas, J.R.Harris, Ancient Egyptian Materials and Industries, Edward Arnold Publishers Ltd., London, 4th edition, 1962
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Website address 1 Comment: Pigments Through the Ages - http://webexhibits.org/pigments/indiv/overview/malachite.html
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: density=3.7-4.1