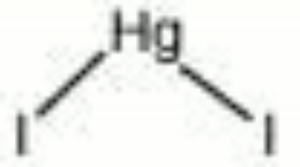
Mercuric iodide
Jump to navigation
Jump to search
Description
A dense, bright red powder that is used as a paint preservative. Mercuric iodide was used for a short period in the early 19th century as a red pigment but it fades to yellow in sunlight.
Synonyms and Related Terms
mercury (II) iodide; red mercury iodide; iodine scarlet; royal scarlet; brilliant scarlet; pure scarlet
Other Properties
Soluble in hot ethanol, acetone and ethyl acetate. Slightly soluble in ether, chloroform, carbon disulfide. Insoluble in water.
| Composition | HgI2 |
|---|---|
| CAS | 7774-29-0 |
| Melting Point | 259 |
| Density | 6.28 |
| Molecular Weight | mol. wt. = 454.399 |
| Refractive Index | 2.748, 2.455 |
| Boiling Point | ~350 |
Hazards and Safety
Highly toxic by ingestion, inhalation and skin absorption. Sensitive to light.
Fisher Scientific: MSDS
Sources Checked for Data in Record
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 5932
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: 'Pigment'
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=2.748, 2.455