Difference between revisions of "Nitrobenzene"
Jump to navigation
Jump to search
(username removed) |
(username removed) |
||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
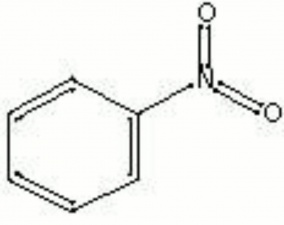
| − | A pale yellow, viscous liquid formed by the reaction of fuming [http://cameo.mfa.org/materials/fullrecord.asp?name=nitric | + | A pale yellow, viscous liquid formed by the reaction of fuming [http://cameo.mfa.org/materials/fullrecord.asp?name=nitric%20acid nitric acid] on [http://cameo.mfa.org/materials/fullrecord.asp?name=benzene benzene]. Nitrobenzene was discovered by Mitscherlich in 1834. When cooled, it forms bright yellow crystals. Nitrobenzene is very toxic. It is used in the manufacture of [http://cameo.mfa.org/materials/fullrecord.asp?name=aniline%20dye aniline dyes]. as well as being an ingredient in [http://cameo.mfa.org/materials/fullrecord.asp?name=soap soaps], [http://cameo.mfa.org/materials/fullrecord.asp?name=polish%20%28material%29 polishes] (metal and shoe), and [http://cameo.mfa.org/materials/fullrecord.asp?name=lubricant lubricating oils]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 47: | Line 47: | ||
== Authority == | == Authority == | ||
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 |
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6685 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6685 | ||
| − | * | + | * F. Crace-Calvert, ''Dyeing and Calico Printing'', Palmer & Howe, London, 1876 Comment: p. 355 |
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.550 | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.550 | ||
Revision as of 07:32, 24 July 2013
Description
A pale yellow, viscous liquid formed by the reaction of fuming nitric acid on benzene. Nitrobenzene was discovered by Mitscherlich in 1834. When cooled, it forms bright yellow crystals. Nitrobenzene is very toxic. It is used in the manufacture of aniline dyes. as well as being an ingredient in soaps, polishes (metal and shoe), and lubricating oils.
Synonyms and Related Terms
oil of mirbane; myrbane; nitrobenzol; essence of mirbane
Other Properties
Soluble in ethanol, benzene, ether, and oils. Slightly soluble in water.
| Composition | C6H5NO2 |
|---|---|
| CAS | 98-95-3 |
| Melting Point | 5.7 |
| Density | 1.19867 |
| Molecular Weight | mol. wt.=123.11 |
| Refractive Index | 1.550 |
| Boiling Point | 210-211 |
Hazards and Safety
Toxic by inhalation, ingestion and skin contact.
Combustible. Flash point = 88 C (190 F)
Mallinckrodt Baker: MSDS
Authority
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 6685
- F. Crace-Calvert, Dyeing and Calico Printing, Palmer & Howe, London, 1876 Comment: p. 355
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index=1.550