Difference between revisions of "Oxalic acid"
(username removed) |
(username removed) |
||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | A colorless, crystalline powder that is a strong organic acid. Oxalic acid was first discovered in the extract from the wood sorrel plant. It also occurs naturally in rhubarb, spinach, beet leaves, Swiss chard, chocolate, cabbage, sweet potatoes, peanuts, cranberries, strawberries, and bell peppers. Additionally, several species of aerobic bacteria (Penicillium and Aspergillus), [http://cameo.mfa.org/materials/fullrecord.asp?name=mold | + | A colorless, crystalline powder that is a strong organic acid. Oxalic acid was first discovered in the extract from the wood sorrel plant. It also occurs naturally in rhubarb, spinach, beet leaves, Swiss chard, chocolate, cabbage, sweet potatoes, peanuts, cranberries, strawberries, and bell peppers. Additionally, several species of aerobic bacteria (Penicillium and Aspergillus), [http://cameo.mfa.org/materials/fullrecord.asp?name=mold%20%28fungus%29 molds], and [http://cameo.mfa.org/materials/fullrecord.asp?name=lichen lichen] excrete oxalic acid as a metabolic product. It is also a component in [http://cameo.mfa.org/materials/fullrecord.asp?name=air%20pollution air pollution] and [http://cameo.mfa.org/materials/fullrecord.asp?name=acid%20rain acid rain]. Oxalic acid is synthetically made from [http://cameo.mfa.org/materials/fullrecord.asp?name=sawdust sawdust] or by treating [http://cameo.mfa.org/materials/fullrecord.asp?name=carbon%20monoxide carbon monoxide] with [http://cameo.mfa.org/materials/fullrecord.asp?name=sodium%20hydroxide sodium hydroxide]. Oxalic acid has many roles as a bleach, metal polish, stain remover, and mordant. It is used as a [http://cameo.mfa.org/materials/fullrecord.asp?name=bleaching%20agent bleaching agent] for [http://cameo.mfa.org/materials/fullrecord.asp?name=straw straw] hats, [http://cameo.mfa.org/materials/fullrecord.asp?name=leather leather], and [http://cameo.mfa.org/materials/fullrecord.asp?name=wood wood]. Oxalic acid acts as a reducing agent for metal oxides to remove tarnish as well as rust or ink stains. It is used commercially as a laundry rinse, wood-bleaching agent, and calcium remover. Oxalic acid can remove some paints and varnishes. It is also used as a mordant in dyeing textiles and in the manufacture of [http://cameo.mfa.org/materials/fullrecord.asp?name=ink ink]. Oxalic acid was used in the toning solution for black and white photography. Since at least the mid-19th century, oxalic acid has been used on works of art: to remove [http://cameo.mfa.org/materials/fullrecord.asp?name=rust rust] and ink stains as well as to bleach leather, wood, papers, stone, and textiles; and to clean encrustations from wall paintings, ceramics, and sculptures. However, these treatments can leave oxalate residues that are difficult to distinguish from naturally occurring residues due to microbiological growth or air pollution. More recently, oxalic acid has been used as a mild hydrolysis agent in the preparation of natural dyes for HPLC analysis. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | ethane diacid; ethanedioic acid; ethanedionic acid; dicarboxylic acid; acid of sugar; saccharine acid; acid of wood sorrel; dibasic acid; | + | ethane diacid; ethanedioic acid; ethanedionic acid; dicarboxylic acid; acid of sugar; saccharine acid; acid of wood sorrel; dibasic acid; Oxalsäure (Deut.); ácido oxálico (Esp., Port.); acide oxalique (Fr.); oxaalzuur (Ned.); kwas szczawiowy (Pol.); oxalsyra (Sven.) |
[[[SliderGallery rightalign|oxalic acid.jpg~Chemical structure]]] | [[[SliderGallery rightalign|oxalic acid.jpg~Chemical structure]]] | ||
| Line 45: | Line 45: | ||
== Additional Information == | == Additional Information == | ||
| − | E.A.Moffatt, N.T.Adair, and G.S.Young, "The occurrence of oxalates on three Chinese wall paintings" in ''Application of science in examination of works of art'' proceedings of the seminar at Museum of Fine Arts, Boston, September 7-9, 1983, (1985), pp. 234-238. | + | ° E.A.Moffatt, N.T.Adair, and G.S.Young, "The occurrence of oxalates on three Chinese wall paintings" in ''Application of science in examination of works of art'' proceedings of the seminar at Museum of Fine Arts, Boston, September 7-9, 1983, (1985), pp. 234-238. ° T. Stambolev, "Notes on the Removal of Iron Stains from Calcareous Stone," Studies in Conservation, 13, 1968. |
== Authority == | == Authority == | ||
| − | * | + | * S.R.Trotman, E.R. Trotman, ''Textile Analysis'', J.B. Lippincott Company, Philadelphia, 1932 |
| − | * | + | * Palmy Weigle, ''Ancient Dyes for Modern Weavers'', Watson-Guptill Publications, New York, 1974 |
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 565 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | * | + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 7043; pH = 1.3 (0.1 M solution) | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 7043; pH = 1.3 (0.1 M solution) | ||
| Line 69: | Line 69: | ||
* ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: pH = 1.6 (0.1 N solution) | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: pH = 1.6 (0.1 N solution) | ||
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
| − | * | + | * Matt Roberts, Don Etherington, ''Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology'', U.S. Government Printing Office, Washington DC, 1982 |
| − | * | + | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 |
| − | * | + | * Tom Rowland, Noel Riley, ''A-Z Guide to Cleaning, Conserving and Repairing Antiques'', Constable and Co., Ltd., London, 1981 |
| − | * | + | * John and Margaret Cannon, ''Dye Plants and Dyeing'', Herbert Press, London, 1994 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 07:56, 24 July 2013
Description
A colorless, crystalline powder that is a strong organic acid. Oxalic acid was first discovered in the extract from the wood sorrel plant. It also occurs naturally in rhubarb, spinach, beet leaves, Swiss chard, chocolate, cabbage, sweet potatoes, peanuts, cranberries, strawberries, and bell peppers. Additionally, several species of aerobic bacteria (Penicillium and Aspergillus), molds, and lichen excrete oxalic acid as a metabolic product. It is also a component in air pollution and acid rain. Oxalic acid is synthetically made from sawdust or by treating carbon monoxide with sodium hydroxide. Oxalic acid has many roles as a bleach, metal polish, stain remover, and mordant. It is used as a bleaching agent for straw hats, leather, and wood. Oxalic acid acts as a reducing agent for metal oxides to remove tarnish as well as rust or ink stains. It is used commercially as a laundry rinse, wood-bleaching agent, and calcium remover. Oxalic acid can remove some paints and varnishes. It is also used as a mordant in dyeing textiles and in the manufacture of ink. Oxalic acid was used in the toning solution for black and white photography. Since at least the mid-19th century, oxalic acid has been used on works of art: to remove rust and ink stains as well as to bleach leather, wood, papers, stone, and textiles; and to clean encrustations from wall paintings, ceramics, and sculptures. However, these treatments can leave oxalate residues that are difficult to distinguish from naturally occurring residues due to microbiological growth or air pollution. More recently, oxalic acid has been used as a mild hydrolysis agent in the preparation of natural dyes for HPLC analysis.
Synonyms and Related Terms
ethane diacid; ethanedioic acid; ethanedionic acid; dicarboxylic acid; acid of sugar; saccharine acid; acid of wood sorrel; dibasic acid; Oxalsäure (Deut.); ácido oxálico (Esp., Port.); acide oxalique (Fr.); oxaalzuur (Ned.); kwas szczawiowy (Pol.); oxalsyra (Sven.)
Other Properties
Soluble in water, glycerol and boiling ethanol. Insoluble in benzene, ligroin, chloroform.
pH = 1.3 (0.1 M solution) pKa=1.27 and 4.27
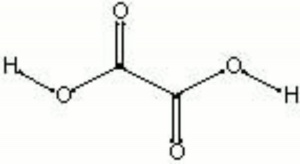
| Composition | HOOCCOOH |
|---|---|
| CAS | 144-62-7 |
| Melting Point | 189.5 (dec) |
| Density | 1.653-1.77 |
| Molecular Weight | mol. wt.= 90.04 |
| Boiling Point | 157 (sublimes) |
Hazards and Safety
Toxic by inhalation and ingestion. Contact causes irritation and corrosion to skin, eyes and membranes. Combustible.
LINK: International Chemical Safety Card
Additional Information
° E.A.Moffatt, N.T.Adair, and G.S.Young, "The occurrence of oxalates on three Chinese wall paintings" in Application of science in examination of works of art proceedings of the seminar at Museum of Fine Arts, Boston, September 7-9, 1983, (1985), pp. 234-238. ° T. Stambolev, "Notes on the Removal of Iron Stains from Calcareous Stone," Studies in Conservation, 13, 1968.
Authority
- S.R.Trotman, E.R. Trotman, Textile Analysis, J.B. Lippincott Company, Philadelphia, 1932
- Palmy Weigle, Ancient Dyes for Modern Weavers, Watson-Guptill Publications, New York, 1974
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 565
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 7043; pH = 1.3 (0.1 M solution)
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Oxalic_acid (Accessed Nov. 9, 2005)
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: pH = 1.6 (0.1 N solution)
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Tom Rowland, Noel Riley, A-Z Guide to Cleaning, Conserving and Repairing Antiques, Constable and Co., Ltd., London, 1981
- John and Margaret Cannon, Dye Plants and Dyeing, Herbert Press, London, 1994