Difference between revisions of "Phenylenediamine"
Jump to navigation
Jump to search
| Line 6: | Line 6: | ||
p-diaminobenzene; 1,4-benzenediamine; o-phenylenediamine; CI 76076 | p-diaminobenzene; 1,4-benzenediamine; o-phenylenediamine; CI 76076 | ||
| − | |||
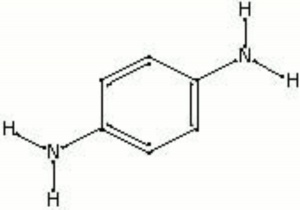
[[[SliderGallery rightalign|phenylenediamine.jpg~Chemical structure]]] | [[[SliderGallery rightalign|phenylenediamine.jpg~Chemical structure]]] | ||
== Risks == | == Risks == | ||
| − | Toxic by ingestion and inhalation. Strongly irritating to skin. | + | * Toxic by ingestion and inhalation. |
| − | + | * Strongly irritating to skin. | |
| − | Fisher Scientific: [https://fscimage.fishersci.com/msds/96738.htm MSDS] | + | * Fisher Scientific: [https://fscimage.fishersci.com/msds/96738.htm MSDS] |
== Physical and Chemical Properties == | == Physical and Chemical Properties == | ||
| − | Soluble in water, ethanol, chloroform and ether. | + | * Soluble in water, ethanol, chloroform and ether. |
| − | + | * Turns black in contact with peroxide; turns brown on contact with iron chloride. | |
| − | Turns black in contact with peroxide; turns brown on contact with iron chloride. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 28: | Line 26: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 145-147 | + | | 145-147 C |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 32: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 267 | + | | 267 C |
|} | |} | ||
Latest revision as of 08:34, 22 October 2022
Description
White to pink crystals that darken with exposure to air. Phenylenediamine is an aromatic compound that is used as a photographic developer and as a stabilizer for direct dyes.
Synonyms and Related Terms
p-diaminobenzene; 1,4-benzenediamine; o-phenylenediamine; CI 76076
Risks
- Toxic by ingestion and inhalation.
- Strongly irritating to skin.
- Fisher Scientific: MSDS
Physical and Chemical Properties
- Soluble in water, ethanol, chloroform and ether.
- Turns black in contact with peroxide; turns brown on contact with iron chloride.
| Composition | C6H4(NH2)2 |
|---|---|
| CAS | 106-50-3 |
| Melting Point | 145-147 C |
| Molecular Weight | mol. wt.=108.14 |
| Boiling Point | 267 C |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entries, 7437, 7438, 7439
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979