Difference between revisions of "Rubber (natural, vulcanized)"
(username removed) |
|||
| (15 intermediate revisions by 6 users not shown) | |||
| Line 4: | Line 4: | ||
A natural hydrocarbon polymer formed from the latex of Euphorbiaceae trees, such as ''Hevea brasiliensis'' or ''Parthenium argentatum''. To prepare natural rubber, the latex is collected from a cut in the bark, precipitated with acid, then washed and dried. Rubber is very elastic and was used for bowls, shoe soles, adhesives and bouncy balls. However, when cooled, rubber becomes brittle and when warmed it becomes sweaty and tacky. Prior to the development of synthetic resins, unvulcanized rubber was used for adhesive tapes and crepe shoe soles. | A natural hydrocarbon polymer formed from the latex of Euphorbiaceae trees, such as ''Hevea brasiliensis'' or ''Parthenium argentatum''. To prepare natural rubber, the latex is collected from a cut in the bark, precipitated with acid, then washed and dried. Rubber is very elastic and was used for bowls, shoe soles, adhesives and bouncy balls. However, when cooled, rubber becomes brittle and when warmed it becomes sweaty and tacky. Prior to the development of synthetic resins, unvulcanized rubber was used for adhesive tapes and crepe shoe soles. | ||
| − | In 1839, Charles Goodyear discovered that rubber can be hardened with the [ | + | In 1839, Charles Goodyear discovered that rubber can be hardened with the [[vulcanization]] process in which [[sulfur]] is used to initiate crosslinking of the hydrocarbon strands. Higher sulfur content produces a harder, denser material. Vulcanized rubber is used to make rubber bands, foams, fabric coatings, small objects, combs, pens and musical instruments. Vulcanized rubber, however, will emit sulfur when exposed to light or heat causing the rubber to degrade and become brittle. Since the 19th century, small amounts of [[wax]] have been added to the rubber during vulcanization. The wax slowly migrates to the surface and provides a thin layer of protection from [[oxidation]]. |
| + | |||
| + | See also [[Rubber (synthetic)|rubber, synthetic]] | ||
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | caucho (Esp.); caoutchouc naturel (Fr.); gomma naturale (It.); borracha natural (Port.); Vulcanite; Ebonite; natural rubber; ''Hevea brasiliensis''; ''Parthenium argentatum'' | + | Latex; India rubber; caoutcchouc; caucho (Esp.); caoutchouc naturel (Fr.); gomma naturale (It.); borracha natural (Port.); Vulcanite; Ebonite; natural rubber; ''Hevea brasiliensis''; ''Parthenium argentatum'' |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
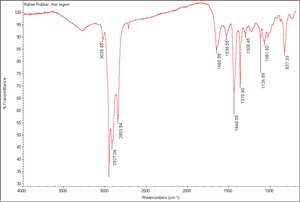
| − | + | [[[SliderGallery rightalign|Native Rubber, thin region.TIF~FTIR(MFA)]]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | == | + | == Applications == |
| + | Historical applications e.g. gasketing, shock mounts | ||
| + | == Personal Risks == | ||
| + | == Collection Risks == | ||
Vulcanized rubber may emit sulfur fumes that will tarnish metals and stain organic materials. | Vulcanized rubber may emit sulfur fumes that will tarnish metals and stain organic materials. | ||
| + | == Environmental Risks == | ||
| + | == Physical and Chemical Properties == | ||
| − | = | + | * Burns with dark yellow, sooty flame; smells of burnt rubber |
| + | * Nonvulcanized rubber becomes brittle at cold temperatures (-34 C) and weakens when heated (66 C). | ||
| + | * Vulcanized rubber degrades at temperatures above 93 C. | ||
| + | * Density = 0.92-1.0 | ||
| + | * Spot test for vulcanized rubber: Iodine/sodium azide reagent for presence of reducible sulfur compounds - positive reaction generates bubbles (Daniels and Ward, 1982) | ||
| − | + | == Working Properties == | |
| + | == Forms and Sizes == | ||
| + | == Resources and Citations == | ||
| − | + | * M.Baker, E.McManus, "History, Care and Handling of America's Spacesuits" JAIC 31:77-85 1992 | |
| − | * | + | * V.Daniels, S.Ward, "A Rapid Test for the Detection of Substances which will Tarnish Silver" ''Studies in Conservation'' 27:58-60, 1982. |
| − | * '' | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 674 |
| − | * | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "rubber" Encyclopædia Britannica [Accessed February 13, 2002] |
| − | * | + | * Pam Hatchfield, ''Pollutants in the Museum Environment'', Archetype Press, London, 2002 |
| − | * | + | * Marjorie Shelley, ''The Care and Handling of Art Objects'', The Metropolitan Museum, New York, 1987 |
| − | * | + | * Rosalie Rosso King, ''Textile Identification, Conservation, and Preservation'', Noyes Publications, Park Ridge, NJ, 1985 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
| − | * | + | * Edward Reich, Carlton J. Siegler, ''Consumer Goods: How to Know and Use Them'', American Book Company, New York City, 1937 |
| − | * | + | * Theodore J. Reinhart, 'Glossary of Terms', ''Engineered Plastics'', ASM International, 1988 |
* Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | * Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000 | ||
| − | * | + | * Website address 1 Comment: www.nswpmith.com.au/historyofplastics.html |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 10:03, 21 July 2020
Description
A natural hydrocarbon polymer formed from the latex of Euphorbiaceae trees, such as Hevea brasiliensis or Parthenium argentatum. To prepare natural rubber, the latex is collected from a cut in the bark, precipitated with acid, then washed and dried. Rubber is very elastic and was used for bowls, shoe soles, adhesives and bouncy balls. However, when cooled, rubber becomes brittle and when warmed it becomes sweaty and tacky. Prior to the development of synthetic resins, unvulcanized rubber was used for adhesive tapes and crepe shoe soles.
In 1839, Charles Goodyear discovered that rubber can be hardened with the Vulcanization process in which Sulfur is used to initiate crosslinking of the hydrocarbon strands. Higher sulfur content produces a harder, denser material. Vulcanized rubber is used to make rubber bands, foams, fabric coatings, small objects, combs, pens and musical instruments. Vulcanized rubber, however, will emit sulfur when exposed to light or heat causing the rubber to degrade and become brittle. Since the 19th century, small amounts of Wax have been added to the rubber during vulcanization. The wax slowly migrates to the surface and provides a thin layer of protection from Oxidation.
See also rubber, synthetic
Synonyms and Related Terms
Latex; India rubber; caoutcchouc; caucho (Esp.); caoutchouc naturel (Fr.); gomma naturale (It.); borracha natural (Port.); Vulcanite; Ebonite; natural rubber; Hevea brasiliensis; Parthenium argentatum
Applications
Historical applications e.g. gasketing, shock mounts
Personal Risks
Collection Risks
Vulcanized rubber may emit sulfur fumes that will tarnish metals and stain organic materials.
Environmental Risks
Physical and Chemical Properties
- Burns with dark yellow, sooty flame; smells of burnt rubber
- Nonvulcanized rubber becomes brittle at cold temperatures (-34 C) and weakens when heated (66 C).
- Vulcanized rubber degrades at temperatures above 93 C.
- Density = 0.92-1.0
- Spot test for vulcanized rubber: Iodine/sodium azide reagent for presence of reducible sulfur compounds - positive reaction generates bubbles (Daniels and Ward, 1982)
Working Properties
Forms and Sizes
Resources and Citations
- M.Baker, E.McManus, "History, Care and Handling of America's Spacesuits" JAIC 31:77-85 1992
- V.Daniels, S.Ward, "A Rapid Test for the Detection of Substances which will Tarnish Silver" Studies in Conservation 27:58-60, 1982.
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 674
- Encyclopedia Britannica, http://www.britannica.com Comment: "rubber" Encyclopædia Britannica [Accessed February 13, 2002]
- Pam Hatchfield, Pollutants in the Museum Environment, Archetype Press, London, 2002
- Marjorie Shelley, The Care and Handling of Art Objects, The Metropolitan Museum, New York, 1987
- Rosalie Rosso King, Textile Identification, Conservation, and Preservation, Noyes Publications, Park Ridge, NJ, 1985
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Edward Reich, Carlton J. Siegler, Consumer Goods: How to Know and Use Them, American Book Company, New York City, 1937
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000
- Website address 1 Comment: www.nswpmith.com.au/historyofplastics.html