Difference between revisions of "Rutile"
| (12 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
| + | [[File:Rutilated quartz-SC268387 (1).jpg|thumb|Rutilated quartz<br>MFA# 01.7556]] | ||
[[File:Gemrutilekes.jpg|thumb|Rutilated quartz pendant]] | [[File:Gemrutilekes.jpg|thumb|Rutilated quartz pendant]] | ||
| − | |||
== Description == | == Description == | ||
| Line 7: | Line 7: | ||
The anatase form of titanium dioxide was a common white pigment in the early 20th century; however, anatase will transform to rutile on heating, and despite efforts to control this during the calcination step of pigment production, some early anatase pigments may have contained up to 20% of coarse, gritty rutile. The white synthetic rutile pigment has significantly higher hiding power and greater resistance to chalking and discoloration on exposure to light than does the anatase pigment. Although experimental rutile pigments were patented in 1931 in Czechoslovakia, Germany and the United States, the first commercially viable methods for production were developed in 1937, based on the sulfate process, which used sulfuric acid to digest ilmenite or titaniferous slag. Industrial production began in 1938/39; in Europe, production was interrupted by World War II, and it was not until after 1945 that rutile pigment became available. The range of composition of the early white rutile pigments varied considerably, as various additives were used to tailor the pigment properties to specific end uses, including compounds of antimony, zinc, tin, magnesium, silicon and lithium, as well as anatase in some cases (to reduce cost). American products from 1939 on included: pure rutile (e.g., Titanox A-NC, coated with 1-2% aluminum oxide and silicon dioxide, manufactured until 1945); composites (e.g., Titanox RC grades, 30% titanium dioxide with calcium sulfate); and adulterations (e.g., Titanox M, 30% titanium dioxide with magnesium silicate; also titanated lithopones with 15% titanium dioxide). | The anatase form of titanium dioxide was a common white pigment in the early 20th century; however, anatase will transform to rutile on heating, and despite efforts to control this during the calcination step of pigment production, some early anatase pigments may have contained up to 20% of coarse, gritty rutile. The white synthetic rutile pigment has significantly higher hiding power and greater resistance to chalking and discoloration on exposure to light than does the anatase pigment. Although experimental rutile pigments were patented in 1931 in Czechoslovakia, Germany and the United States, the first commercially viable methods for production were developed in 1937, based on the sulfate process, which used sulfuric acid to digest ilmenite or titaniferous slag. Industrial production began in 1938/39; in Europe, production was interrupted by World War II, and it was not until after 1945 that rutile pigment became available. The range of composition of the early white rutile pigments varied considerably, as various additives were used to tailor the pigment properties to specific end uses, including compounds of antimony, zinc, tin, magnesium, silicon and lithium, as well as anatase in some cases (to reduce cost). American products from 1939 on included: pure rutile (e.g., Titanox A-NC, coated with 1-2% aluminum oxide and silicon dioxide, manufactured until 1945); composites (e.g., Titanox RC grades, 30% titanium dioxide with calcium sulfate); and adulterations (e.g., Titanox M, 30% titanium dioxide with magnesium silicate; also titanated lithopones with 15% titanium dioxide). | ||
| + | [[File:Rutilekes.jpg|thumb|Rultile mineral]] | ||
Although research to produce pigment from titanium tetrachloride had been underway since the 1920s, it was only when large quantities of chlorine were available to manufacturers that a pilot plant (E.I. du Pont de Nemours and Co. in the United States) first produced pigment in 1948 using the chloride process. In this method, chlorine gas is reacted with an ore of high titanium dioxide content (generally rutile or synthetic rutile) followed by vapor phase oxidation. Anatase could also be produced using this method; however, it contained a significant amount of rutile and was only briefly offered. Because of differences in the manufacturing methods, sulfate process rutile can possibly be distinguished from the purer chloride process rutile on the basis of trace elements such as niobium, vanadium, chromium, zirconium and tantalum, although it can be difficult to distinguish the purer grades. Elevated levels of lead may also be present in sulfate process titanium dioxide pigments, due to leaching from pipes and tanks during manufacture. | Although research to produce pigment from titanium tetrachloride had been underway since the 1920s, it was only when large quantities of chlorine were available to manufacturers that a pilot plant (E.I. du Pont de Nemours and Co. in the United States) first produced pigment in 1948 using the chloride process. In this method, chlorine gas is reacted with an ore of high titanium dioxide content (generally rutile or synthetic rutile) followed by vapor phase oxidation. Anatase could also be produced using this method; however, it contained a significant amount of rutile and was only briefly offered. Because of differences in the manufacturing methods, sulfate process rutile can possibly be distinguished from the purer chloride process rutile on the basis of trace elements such as niobium, vanadium, chromium, zirconium and tantalum, although it can be difficult to distinguish the purer grades. Elevated levels of lead may also be present in sulfate process titanium dioxide pigments, due to leaching from pipes and tanks during manufacture. | ||
| Line 13: | Line 14: | ||
titanium dioxide; synthetic rutile; imitation diamond; rutile (Eng., Fr.); rutilo (It., Esp., Port.); rutil (Nor., Sven.); ; Titanweiss (Rutil) (Deut.); leyko toy titanioy (Roytilio) (Gr.); bianco di titanio (rutilo) (It.); titaandioxide (rutiel) (Ned.); rútilo (Port.) | titanium dioxide; synthetic rutile; imitation diamond; rutile (Eng., Fr.); rutilo (It., Esp., Port.); rutil (Nor., Sven.); ; Titanweiss (Rutil) (Deut.); leyko toy titanioy (Roytilio) (Gr.); bianco di titanio (rutilo) (It.); titaandioxide (rutiel) (Ned.); rútilo (Port.) | ||
| − | [[[SliderGallery rightalign|Rutileitaly1.jpg~Raman| | + | Commericial diamond initations: Astryl; Diamothyst; Gava or Java Gem; Meredith; Miridis; Rainbow Diamond; Rainbow Magic Diamond; Rutania; Titangem; Titania; Ultamite |
| − | + | == Risks == | |
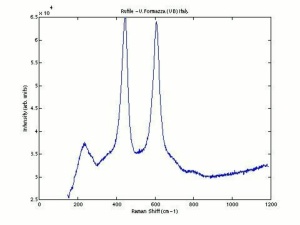
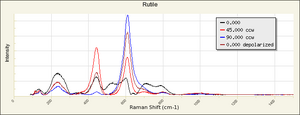
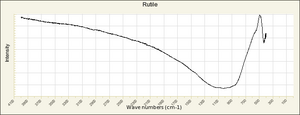
| − | == | + | [[[SliderGallery rightalign|Rutileitaly1.jpg~Raman (U of Parma)|Rutile Raman RRUFF R050031.png~Raman (RRUFF)|Rutile IR-ATR RRUFF R050031.png~IR-ATR (RRUFF)]]] |
| − | + | Nontoxic. No significant hazards. | |
| − | + | == Physical and Chemical Properties == | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * Tetragonal crystal system. | |
| − | + | * Optic sign uniaxial, positive. | |
| − | Chemically inert, insoluble in water, organic solvents, aqueous alkalis; can be dissolved in sulfuric or hydrofluoric acid; slow to dry in oil without additives or surface treatment. | + | * Particle size of modern pure rutile pigment 0.2 - 0.23 micrometers; composites up to 0.8 micrometers |
| − | + | * Fluorescence = inert to weak | |
| − | Absorbs strongly in the UV, is photochemically active but less so than anatase titanium dioxide. | + | * Birefringence = high (0.287) under crossed polars |
| + | * Chemically inert, insoluble in water, organic solvents, aqueous alkalis; can be dissolved in sulfuric or hydrofluoric acid; slow to dry in oil without additives or surface treatment. | ||
| + | * Absorbs strongly in the UV, is photochemically active but less so than anatase titanium dioxide. | ||
| + | * Synthetic stones show much more fire than diamonds and exhibit visible doubling; the faceted edges may also show abrasion | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 40: | Line 38: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | | + | | 1825 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| Line 48: | Line 46: | ||
| 2.71 - 2.72 (mineral); 1.98 (e.g., Titanox RC composite) | | 2.71 - 2.72 (mineral); 1.98 (e.g., Titanox RC composite) | ||
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| − | [[media: | + | [[media:download_file_413.pdf|Natural and Simulated Diamonds]] |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | [[media:download_file_517.pdf|Characteristics of Common White Pigments]] | |
| + | ==Resources and Citations== | ||
| + | * Gem Identification Lab Manual, Gemological Institute of America, 2016. | ||
| + | * M.Laver, "Titanium Dioxide Whites", ''Artists Pigments'', Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997. | ||
| + | * Mineralogy Database: [http://www.webmineral.com/data/Rutile.shtml Rutile] | ||
| + | * Walter C. McCrone, "Polarized Light Microscopy in Conservation: A Personal Perspective" ''JAIC'' 33(2):101-14, 1994. (contains a table of dates on the history of titanium white as a pigment) | ||
* Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 | ||
| − | + | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density=4.2 and ref.index=2.9, 2.6 | |
| − | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density=4.2 and ref.index | ||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 815 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 815 | ||
| − | |||
* Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | * Ralph Mayer, ''A Dictionary of Art Terms and Techniques'', Harper and Row Publishers, New York, 1969 (also 1945 printing) | ||
| − | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "rutile" [Accessed January 22, 2002]. | |
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "rutile" | ||
| − | |||
* C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | * C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | ||
| − | |||
* Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 Comment: ref. index = 2.61; 1.90; | * Thomas B. Brill, ''Light Its Interaction with Art and Antiquities'', Plenum Press, New York City, 1980 Comment: ref. index = 2.61; 1.90; | ||
| − | + | * Wikipedia: http://en.wikipedia.org/wiki/Rutile (Accessed Sept. 14, 2005) | |
| − | * Wikipedia | ||
| − | |||
* ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 | ||
| − | |||
* Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
| − | |||
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
Latest revision as of 10:55, 4 January 2023
Description
There are three naturally occurring isomorphic forms of Titanium dioxide: Anatase, rutile, and Brookite. Naturally occurring rutile can vary considerably in shape and in color (from yellow to brownish yellow, red, brown-red, brown, bluish or violet, rarely green, to greyish-black or black, due to various elemental impurities). Mineral rutile is mined from beach sand in Australia, and productive deposits also occur in Sierra Leone, South Africa, the United States, India, Norway, Sri Lanka, and Brazil. Small amounts of natural rutile may have been used as a pale yellow to red brown or nearly black pigment; however, it is difficult to grind and produces coarse, angular particles much different than the modern white pigment in color and morphology, and higher in impurities. Synthetic rutile gemstone crystals were first prepared in 1948 using the Verneuil process and can be produced in different colours depending on additives. Rutile is used as a coating in the manufacture of welding rods.
The anatase form of titanium dioxide was a common white pigment in the early 20th century; however, anatase will transform to rutile on heating, and despite efforts to control this during the calcination step of pigment production, some early anatase pigments may have contained up to 20% of coarse, gritty rutile. The white synthetic rutile pigment has significantly higher hiding power and greater resistance to chalking and discoloration on exposure to light than does the anatase pigment. Although experimental rutile pigments were patented in 1931 in Czechoslovakia, Germany and the United States, the first commercially viable methods for production were developed in 1937, based on the sulfate process, which used sulfuric acid to digest ilmenite or titaniferous slag. Industrial production began in 1938/39; in Europe, production was interrupted by World War II, and it was not until after 1945 that rutile pigment became available. The range of composition of the early white rutile pigments varied considerably, as various additives were used to tailor the pigment properties to specific end uses, including compounds of antimony, zinc, tin, magnesium, silicon and lithium, as well as anatase in some cases (to reduce cost). American products from 1939 on included: pure rutile (e.g., Titanox A-NC, coated with 1-2% aluminum oxide and silicon dioxide, manufactured until 1945); composites (e.g., Titanox RC grades, 30% titanium dioxide with calcium sulfate); and adulterations (e.g., Titanox M, 30% titanium dioxide with magnesium silicate; also titanated lithopones with 15% titanium dioxide).
Although research to produce pigment from titanium tetrachloride had been underway since the 1920s, it was only when large quantities of chlorine were available to manufacturers that a pilot plant (E.I. du Pont de Nemours and Co. in the United States) first produced pigment in 1948 using the chloride process. In this method, chlorine gas is reacted with an ore of high titanium dioxide content (generally rutile or synthetic rutile) followed by vapor phase oxidation. Anatase could also be produced using this method; however, it contained a significant amount of rutile and was only briefly offered. Because of differences in the manufacturing methods, sulfate process rutile can possibly be distinguished from the purer chloride process rutile on the basis of trace elements such as niobium, vanadium, chromium, zirconium and tantalum, although it can be difficult to distinguish the purer grades. Elevated levels of lead may also be present in sulfate process titanium dioxide pigments, due to leaching from pipes and tanks during manufacture.
Synonyms and Related Terms
titanium dioxide; synthetic rutile; imitation diamond; rutile (Eng., Fr.); rutilo (It., Esp., Port.); rutil (Nor., Sven.); ; Titanweiss (Rutil) (Deut.); leyko toy titanioy (Roytilio) (Gr.); bianco di titanio (rutilo) (It.); titaandioxide (rutiel) (Ned.); rútilo (Port.)
Commericial diamond initations: Astryl; Diamothyst; Gava or Java Gem; Meredith; Miridis; Rainbow Diamond; Rainbow Magic Diamond; Rutania; Titangem; Titania; Ultamite
Risks
Nontoxic. No significant hazards.
Physical and Chemical Properties
- Tetragonal crystal system.
- Optic sign uniaxial, positive.
- Particle size of modern pure rutile pigment 0.2 - 0.23 micrometers; composites up to 0.8 micrometers
- Fluorescence = inert to weak
- Birefringence = high (0.287) under crossed polars
- Chemically inert, insoluble in water, organic solvents, aqueous alkalis; can be dissolved in sulfuric or hydrofluoric acid; slow to dry in oil without additives or surface treatment.
- Absorbs strongly in the UV, is photochemically active but less so than anatase titanium dioxide.
- Synthetic stones show much more fire than diamonds and exhibit visible doubling; the faceted edges may also show abrasion
| Composition | TiO2; common impurities (mineral) Si, Al, Fe, Ta, Nb, Zr, Cr, V, Sn |
|---|---|
| Mohs Hardness | 6.0 - 6.5 |
| Melting Point | 1825 C |
| Density | 4.25 (calc.); 3.75 (coated pigment)- 4.2 (mineral) |
| Refractive Index | 2.71 - 2.72 (mineral); 1.98 (e.g., Titanox RC composite) |
Comparisons
Natural and Simulated Diamonds
Characteristics of Common White Pigments
Resources and Citations
- Gem Identification Lab Manual, Gemological Institute of America, 2016.
- M.Laver, "Titanium Dioxide Whites", Artists Pigments, Volume 3, E. West FitzHugh (ed.), Oxford University Press: Oxford, 1997.
- Mineralogy Database: Rutile
- Walter C. McCrone, "Polarized Light Microscopy in Conservation: A Personal Perspective" JAIC 33(2):101-14, 1994. (contains a table of dates on the history of titanium white as a pigment)
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density=4.2 and ref.index=2.9, 2.6
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 815
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- Encyclopedia Britannica, http://www.britannica.com Comment: "rutile" [Accessed January 22, 2002].
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980 Comment: ref. index = 2.61; 1.90;
- Wikipedia: http://en.wikipedia.org/wiki/Rutile (Accessed Sept. 14, 2005)
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998