Difference between pages "Sodium azide test" and "Sodium benzoate"
(Difference between pages)
Jump to navigation
Jump to search
| Line 1: | Line 1: | ||
| − | |||
== Description == | == Description == | ||
| − | + | White granular powder. Sodium benzoate is used as an antiseptic and preservative in paints, food, and medicine. It is also used as a rust and mildew inhibitor. | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | + | benzoate of soda; sodium benzoic acid; Antimol | |
| + | |||
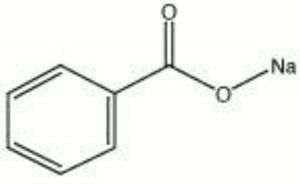
| + | [[[SliderGallery rightalign|sodium benzoate.jpg~Chemical structure]]] | ||
== Risks == | == Risks == | ||
| − | * | + | * Combustible. |
| − | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber= | + | * Ingestion of large quantities is toxic. |
| + | * Maximum allowable concentration in food is 0.1%. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AAA1594630&productDescription=SODIUM+BENZOATE+99%25+250G&vendorId=VN00024248&countryCode=US&language=en SDS] | ||
| + | |||
| + | ==Physical and Chemical Properties== | ||
| + | |||
| + | Soluble in water, ethanol. Forms slightly alkaline solution in water. | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! scope="row"| Composition | ||
| + | | C6H5COONa | ||
| + | |- | ||
| + | ! scope="row"| CAS | ||
| + | | 532-32-1 | ||
| + | |- | ||
| + | ! scope="row"| Molecular Weight | ||
| + | | mol. wt. = 144.1 | ||
| + | |} | ||
==Resources and Citations== | ==Resources and Citations== | ||
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
| + | |||
| + | * Thomas Gregory, ''The Condensed Chemical Dictionary'', Reinhold Publishing, New York, 3rd ed., 1942 | ||
| − | * | + | * ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 8725 |
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p 777 |
| − | * | + | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 |
| − | * '' | + | * ''Van Nostrand's Scientific Encyclopedia'', Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 15:41, 31 May 2022
Description
White granular powder. Sodium benzoate is used as an antiseptic and preservative in paints, food, and medicine. It is also used as a rust and mildew inhibitor.
Synonyms and Related Terms
benzoate of soda; sodium benzoic acid; Antimol
Risks
- Combustible.
- Ingestion of large quantities is toxic.
- Maximum allowable concentration in food is 0.1%.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water, ethanol. Forms slightly alkaline solution in water.
| Composition | C6H5COONa |
|---|---|
| CAS | 532-32-1 |
| Molecular Weight | mol. wt. = 144.1 |
Resources and Citations
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Thomas Gregory, The Condensed Chemical Dictionary, Reinhold Publishing, New York, 3rd ed., 1942
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 8725
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p 777
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976