Difference between pages "Sodium benzoate" and "Sodium bicarbonate"
(Difference between pages)
Jump to navigation
Jump to search
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | White | + | White, lumpy powder that is often used to make effervescent salts and beverages and as a leavening agent for baking. Sodium bicarbonate is also used in fire extinguishers and metal ([[gold|gold]] and [[platinum|platinum]]) plating baths. Sodium bicarbonate readily absorbs stains and odors, it is used in dry-cleaning preparations for textiles, carpet, plastics, and canework. It is also used as a mild abrasive in some scouring powders. Sodium bicarbonate is used in the neutralization step of alum tawing. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | + | baking soda; sodium acid carbonate; sodium hydrogen carbonate; bicarbonate of soda | |
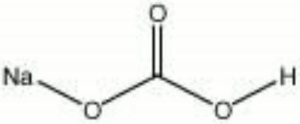
| − | [[[SliderGallery rightalign|sodium | + | [[[SliderGallery rightalign|sodium bicarbonate.jpg~Chemical structure]]] |
== Risks == | == Risks == | ||
| − | * | + | * Fisher Scientific: [https://beta-static.fishersci.com/content/dam/fishersci/en_US/documents/programs/education/regulatory-documents/sds/chemicals/chemicals-s/S25533.pdf SDS] |
| − | |||
| − | |||
| − | |||
==Physical and Chemical Properties== | ==Physical and Chemical Properties== | ||
| − | Soluble in water | + | * Soluble in water. Insoluble in ethanol. |
| + | * For a 0.1 molar solution, the pH = 8.3. | ||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
! scope="row"| Composition | ! scope="row"| Composition | ||
| − | | | + | | NaHCO3 |
|- | |- | ||
! scope="row"| CAS | ! scope="row"| CAS | ||
| − | | | + | | 144-55-8 |
| + | |- | ||
| + | ! scope="row"| Mohs Hardness | ||
| + | | 2.5 | ||
| + | |- | ||
| + | ! scope="row"| Density | ||
| + | | 2.159 g/ml | ||
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| − | | mol. wt. = | + | | mol. wt. = 84.01 |
| + | |- | ||
| + | ! scope="row"| Refractive Index | ||
| + | | 1.376, 1.500, 1.582 | ||
|} | |} | ||
==Resources and Citations== | ==Resources and Citations== | ||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 734 |
| − | * | + | * Hermann Kuhn, ''Conservation and Restoration of Works of Art and Antiquities'', Butterworths, London, 1986 |
| − | * '' | + | * Palmy Weigle, ''Ancient Dyes for Modern Weavers'', Watson-Guptill Publications, New York, 1974 |
| − | * | + | * Photographic chemicals at www.jetcity.com/~mrjones/chemdesc.htm |
| + | |||
| + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 | ||
| + | |||
| + | * Tom Rowland, Noel Riley, ''A-Z Guide to Cleaning, Conserving and Repairing Antiques'', Constable and Co., Ltd., London, 1981 | ||
| + | |||
| + | * R.M.Organ, ''Design for Scientific Conservation of Antiquities'', Smithsonian Institution, Washington DC, 1968 | ||
| + | |||
| + | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
| + | |||
| + | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8726 | ||
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| − | * '' | + | * ''CRC Handbook of Chemistry and Physics'', Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index = 1.376, 1.500, 1.582 |
| + | |||
| + | * Gordon Hanlon, contributed information, 1998 | ||
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 15:44, 31 May 2022
Description
White, lumpy powder that is often used to make effervescent salts and beverages and as a leavening agent for baking. Sodium bicarbonate is also used in fire extinguishers and metal (Gold and Platinum) plating baths. Sodium bicarbonate readily absorbs stains and odors, it is used in dry-cleaning preparations for textiles, carpet, plastics, and canework. It is also used as a mild abrasive in some scouring powders. Sodium bicarbonate is used in the neutralization step of alum tawing.
Synonyms and Related Terms
baking soda; sodium acid carbonate; sodium hydrogen carbonate; bicarbonate of soda
Risks
- Fisher Scientific: SDS
Physical and Chemical Properties
- Soluble in water. Insoluble in ethanol.
- For a 0.1 molar solution, the pH = 8.3.
| Composition | NaHCO3 |
|---|---|
| CAS | 144-55-8 |
| Mohs Hardness | 2.5 |
| Density | 2.159 g/ml |
| Molecular Weight | mol. wt. = 84.01 |
| Refractive Index | 1.376, 1.500, 1.582 |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 734
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- Palmy Weigle, Ancient Dyes for Modern Weavers, Watson-Guptill Publications, New York, 1974
- Photographic chemicals at www.jetcity.com/~mrjones/chemdesc.htm
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Tom Rowland, Noel Riley, A-Z Guide to Cleaning, Conserving and Repairing Antiques, Constable and Co., Ltd., London, 1981
- R.M.Organ, Design for Scientific Conservation of Antiquities, Smithsonian Institution, Washington DC, 1968
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8726
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index = 1.376, 1.500, 1.582
- Gordon Hanlon, contributed information, 1998