Difference between revisions of "Silver sulfide"
Jump to navigation
Jump to search
(username removed) |
(username removed) |
||
| Line 39: | Line 39: | ||
== Authority == | == Authority == | ||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 726 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8677 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8677 | ||
| − | * | + | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Revision as of 07:34, 24 July 2013
Description
A grayish-black powder. Silver sulfide, or silver glance, is an important silver ore. It is used as a black colorant in ceramic glazes and for niello metal work.
Synonyms and Related Terms
silver (I) sulfide; silver sulphide; argentous sulfide; argentite; silver glance; vitreous silver; sulfide of silver
Other Properties
Soluble in concentrated sulfuric and nitric acids. Insoluble in water.
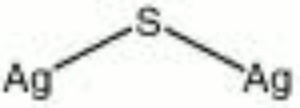
| Composition | Ag2S |
|---|---|
| CAS | 21548-73-2 |
| Melting Point | 825 |
| Density | 7.32 |
| Molecular Weight | mol. wt. = 247.8 |
Hazards and Safety
Sensitive to light. Contact may cause irritation.
Fisher Scientific: MSDS
Authority
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 726
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8677
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985