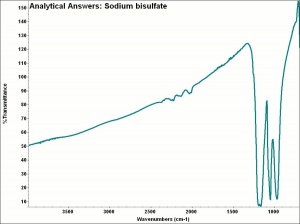
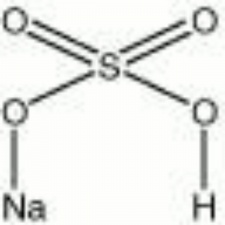
Sodium bisulfate
Jump to navigation
Jump to search
Description
Colorless crystals or white lumps. Sodium bisulfate is used as an inexpensive substitute for Sulfuric acid in dyeing solutions. It is also used in the manufacture of Paper, Soap, magnesia cements, and acid-based industrial cleaners. Sodium bisulfate acts as a Flux in metallurgy. It can pickle metals, carbonize Wool, and bleach Leather.
Synonyms and Related Terms
sodium acid sulfate; niter cake; sodium hydrogen sulfate; sodium bisulphate (Br.); sodium pyrosulfate
Other Properties
Soluble in water forming an acidic solution (0.1 molar has a pH of 1.4). Decomposes in ethanol to form sodium sulfate and sulfuric acid.
| Composition | NaHSO4 |
|---|---|
| CAS | 7681-38-1 |
| Melting Point | 315 |
| Density | 2.435 |
| Molecular Weight | mol. wt. = 120.06 |
| Refractive Index | 1.43, 1.46, 1.47 |
Hazards and Safety
Strongly irritating to skin, eyes and lungs. Hygroscopic. Noncombustible.
Mallinckrodt Baker: MSDS
Sources Checked for Data in Record
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8727
- Website address 1 Comment: www.jetcity.com/~mrjones/chemdesc.htm - photographic chemicals
- CRC Handbook of Chemistry and Physics, Robert Weast (ed.), CRC Press, Boca Raton, Florida, v. 61, 1980 Comment: ref. index = 1.43, 1.46, 1.47
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- MSDS Sheet