Difference between revisions of "Sodium dithionite"
(username removed) |
|||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | Pale yellow powder that is used as a [ | + | Pale yellow powder that is used as a [[reducing%20agent|reducing agent]] in dyeing [[indigo]] and [[vat%20dye vat dyes]]. Sodium dithionite is also used to strip dyes from dyed textiles and reduce [[iron%20stain|iron oxide stains]] to [[ferrous%20oxide ferrous oxide]. Sodium dithionite was also used as a [[bleaching%20agent|bleach]] for [[leather]] and [[mechanical%20wood%20pulp|mechanical paper pulps]] but its use has declined in recent years due to poor color reversion properties (AIC Book and Paper Catalog). |
| − | Note: this is not the same compound as [ | + | Note: this is not the same compound as [[sodium%20thiosulfate|sodium thiosulfate]] (Na2S2O3; also called sodium hyposulfite) that is used for fixation in photography. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
Revision as of 13:32, 4 November 2015
Description
Pale yellow powder that is used as a Reducing agent in dyeing Indigo and Vat dye vat dyes. Sodium dithionite is also used to strip dyes from dyed textiles and reduce iron oxide stains to [[ferrous%20oxide ferrous oxide]. Sodium dithionite was also used as a bleach for Leather and mechanical paper pulps but its use has declined in recent years due to poor color reversion properties (AIC Book and Paper Catalog).
Note: this is not the same compound as Sodium thiosulfate (Na2S2O3; also called sodium hyposulfite) that is used for fixation in photography.
Synonyms and Related Terms
sodium thiosulfite; sodium hydrosulfite; sodium sulfoxylate
Other Properties
Soluble in water (pH = 6.0-7.5 for 1-6% solution). Insoluble in ethanol.
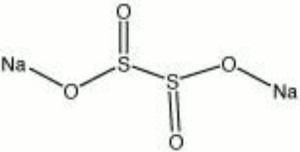
| Composition | Na2S2O4 |
|---|---|
| CAS | 7775-14-6 |
| Melting Point | 52-55 |
| Density | 2.19 |
| Molecular Weight | mol. wt. = 174.1 |
Hazards and Safety
Fire risk in contact with moisture and air. Use dry sand to extinguish fires. Flash point=90 C
Contact causes irritation
Mallinckrodt Baker: MSDS
Additional Information
AIC Book and Paper Catalog, p.
Authority
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 786
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Matt Roberts, Don Etherington, Bookbinding and the Conservation of Books: a Dictionary of Descriptive Terminology, U.S. Government Printing Office, Washington DC, 1982
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- The Dictionary of Paper, American Paper Institute, New York, Fourth Edition, 1980
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8771
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Sodium_dithionite (Accessed Jan. 6 2006)