Difference between revisions of "Stearic acid"
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| (2 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
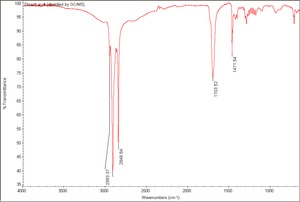
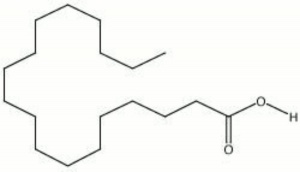
| − | + | [[[SliderGallery rightalign|Stearic acid (identified by GC MS).TIF~FTIR (MFA)|stearic acid.jpg~Chemical structure]]] | |
White, waxy fatty acid. Stearic acid occurs naturally in animal fats, [[tallow|tallow]], and, to a smaller extent, vegetable fats. Lard and tallow can contain up to 30% stearic acid. Stearic acid is a long-chain saturated triglyceride with no double bonds that can be readily saponified with alkaline salts. Most commercial stearic acid products, such as U.S.P. stearic acid, contain are a mixture of 50% stearic acid, 45% [[palmitic%20acid|palmitic acid]], and 5% [[oleic%20acid|oleic acid]]. Stearic acid is used as a lubricatant, softener, and dispersing agent in soaps, candles, lubricants, ointments, cosmetics, rubber, polishes, coatings, and food packaging. | White, waxy fatty acid. Stearic acid occurs naturally in animal fats, [[tallow|tallow]], and, to a smaller extent, vegetable fats. Lard and tallow can contain up to 30% stearic acid. Stearic acid is a long-chain saturated triglyceride with no double bonds that can be readily saponified with alkaline salts. Most commercial stearic acid products, such as U.S.P. stearic acid, contain are a mixture of 50% stearic acid, 45% [[palmitic%20acid|palmitic acid]], and 5% [[oleic%20acid|oleic acid]]. Stearic acid is used as a lubricatant, softener, and dispersing agent in soaps, candles, lubricants, ointments, cosmetics, rubber, polishes, coatings, and food packaging. | ||
| Line 7: | Line 7: | ||
n-octadecanoic acid (IUPAC); Emersol 132; Promulsin; Proviscol Wax. U.S.P. stearic acid; kyselina stearová (Ces.); Stearinsäure (Deut.); ácido esteárico (Esp.); acide stéarique (Fr.); (It.); stearinezuur (Ned.); kwas stearynowy (Pol.); | n-octadecanoic acid (IUPAC); Emersol 132; Promulsin; Proviscol Wax. U.S.P. stearic acid; kyselina stearová (Ces.); Stearinsäure (Deut.); ácido esteárico (Esp.); acide stéarique (Fr.); (It.); stearinezuur (Ned.); kwas stearynowy (Pol.); | ||
| − | + | == Risks == | |
| − | == | + | * Combustible. |
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC174490010&productDescription=STEARIC+ACID%2C+97%2B%25%28GC%29+1KG&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| + | |||
| + | == Physical and Chemical Properties == | ||
Almost insoluble in water. | Almost insoluble in water. | ||
| Line 22: | Line 25: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 69.6 | + | | 69.6 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 0.8390 | + | | 0.8390 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 37: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 361-383 | + | | 361-383 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 770 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 770 | ||
| Line 53: | Line 50: | ||
* ''A Glossary of Paper Conservation Terms'', Margaret Ellis (ed.), Conservation Center of the Institute of Fine Arts, New York City, 1998 | * ''A Glossary of Paper Conservation Terms'', Margaret Ellis (ed.), Conservation Center of the Institute of Fine Arts, New York City, 1998 | ||
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "stearic acid" | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "stearic acid" [Accessed 25 Jan. 2006]. |
| − | * Wikipedia | + | * Wikipedia: http://en.wikipedia.org/wiki/Stearic_acid (Accessed Feb. 10, 2006) |
* Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | * Random House, ''Webster's Encyclopedic Unabridged Dictionary of the English Language'', Grammercy Book, New York, 1997 | ||
Latest revision as of 15:20, 4 June 2022
Description
White, waxy fatty acid. Stearic acid occurs naturally in animal fats, Tallow, and, to a smaller extent, vegetable fats. Lard and tallow can contain up to 30% stearic acid. Stearic acid is a long-chain saturated triglyceride with no double bonds that can be readily saponified with alkaline salts. Most commercial stearic acid products, such as U.S.P. stearic acid, contain are a mixture of 50% stearic acid, 45% Palmitic acid, and 5% Oleic acid. Stearic acid is used as a lubricatant, softener, and dispersing agent in soaps, candles, lubricants, ointments, cosmetics, rubber, polishes, coatings, and food packaging.
Synonyms and Related Terms
n-octadecanoic acid (IUPAC); Emersol 132; Promulsin; Proviscol Wax. U.S.P. stearic acid; kyselina stearová (Ces.); Stearinsäure (Deut.); ácido esteárico (Esp.); acide stéarique (Fr.); (It.); stearinezuur (Ned.); kwas stearynowy (Pol.);
Risks
- Combustible.
- ThermoFisher: SDS
Physical and Chemical Properties
Almost insoluble in water.
| Composition | CH3(CH2)16COOH |
|---|---|
| CAS | 57-11-4 |
| Melting Point | 69.6 C |
| Density | 0.8390 g/ml |
| Molecular Weight | mol. wt. = 284.47 |
| Refractive Index | 1.4299 |
| Boiling Point | 361-383 C |
Resources and Citations
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 770
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 8959
- A Glossary of Paper Conservation Terms, Margaret Ellis (ed.), Conservation Center of the Institute of Fine Arts, New York City, 1998
- Encyclopedia Britannica, http://www.britannica.com Comment: "stearic acid" [Accessed 25 Jan. 2006].
- Wikipedia: http://en.wikipedia.org/wiki/Stearic_acid (Accessed Feb. 10, 2006)
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998