Difference between revisions of "Styrofoam"
Jump to navigation
Jump to search
| Line 10: | Line 10: | ||
== Risks == | == Risks == | ||
| − | Flash point 345C (670F). Combustion products may include carbon dioxide and carbon monoxide. | + | * Flash point 345C (670F). Combustion products may include carbon dioxide and carbon monoxide. |
| − | + | * National Foam: [https://nationalfoam.com/foam-concentrates/foam-concentrate-sds/ SDS] | |
| − | |||
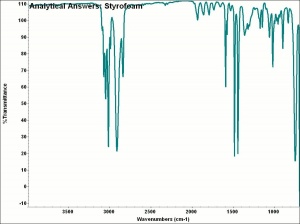
[[[SliderGallery rightalign|aaiSTYROFOM.jpg~FTIR]]] | [[[SliderGallery rightalign|aaiSTYROFOM.jpg~FTIR]]] | ||
| Line 18: | Line 17: | ||
== Resources and Citations == | == Resources and Citations == | ||
| − | * | + | * DuPont: [https://www.dupont.com/brands/styrofoam.html Styrofoam Website] |
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
Latest revision as of 13:16, 6 June 2022
Description
[Dow Chemical Co.] A registered trademark for foamed Polystyrene that was developed in the 1940s. Some formulations of Styrofoam® may contain up to 10% Polyethylene or Polypropylene. The foam is produced with ethyl chloride and chlorodifluoroethane blowing agents. These halogenated gases may cause metal corrosion when Styrofoam® is used as insulation near heat sources. Styrofoam® is available in foamed sheets and extruded pellets and rods. It is primarily used for packing, but has other applications in flotation devices, insulation, and toys.
Synonyms and Related Terms
foamed polystyrene
Risks
- Flash point 345C (670F). Combustion products may include carbon dioxide and carbon monoxide.
- National Foam: SDS
Resources and Citations
- DuPont: Styrofoam Website
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 755
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000