Supercritical fluid
Revision as of 07:21, 24 July 2013 by (username removed)
Description
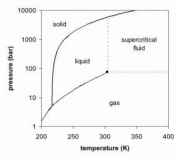
A material compressed and heated to a point above its thermodynamic critical point. Supercritical fluids (SCF) have the unique ability to penetrate materials like a gas while also dissolving materials like a liquid. Carbon dioxide and water are the most commonly used supercritical fluids. A temperatures and pressures above the thermodynamic critical point, a material's liquid phase and the gas phase will have equal densities and are indistinguishable.
Synonyms and Related Terms
SCF
File:.jpg
Table of critical values
Additional Information
° Sung Mo Kang, Achim Unger, J.J. Morrell, 'The Effect of Supercritical Carbon Dioxide Extraction of Color Retention and Pesticide Reduction of Wooden Artifacts' JAIC 43(2) 151-160, 2004.
Authority
- Wikipedia, the free encyclopedia, at http://www.wikipedia.com Comment: http://en.wikipedia.org/wiki/Supercritical_fluid (Accessed Dec. 9, 2005)