Difference between revisions of "Teflon"
| Line 15: | Line 15: | ||
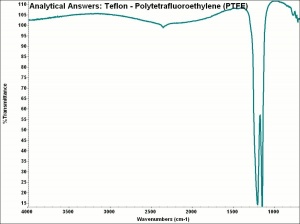
[[[SliderGallery rightalign|aaiTEFLON.jpg~FTIR]]] | [[[SliderGallery rightalign|aaiTEFLON.jpg~FTIR]]] | ||
| − | |||
== Applications == | == Applications == | ||
Revision as of 14:45, 1 December 2019
Description
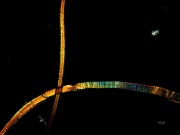
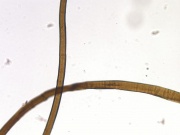
[DuPont] A registered trademark for a solid white polymer that is resistant to almost all chemicals. Polytetrafluoroethylene was accidentally discovered in 1938 by Roy Plunkett at DuPont during an attempt to synthesize tetrafluoroethylene. The new polymer was found to be resistant to heat, chemical, light, and adhesion and subsequently marketed as Teflon® by DuPont in 1943. Teflon® is a soft, opaque material that is unaffected by acids, alkalis, and organic solvents. It is widely used for containers in chemical plants, for rockets, bearings, gaskets, and for frying pan coatings. Teflon® is used for stain-resistant, water-repellent coatings on textiles. It can also be prepared as ribbonlike fibers, which are woven, knitted, felted or braided. Additionally, GORE-TEX® is prepared from a microporous Teflon® film laminated on a polyester fabric.
See also fluorinated ethylene propylene.
Synonyms and Related Terms
PTFE; polytetrafluoroethylene; Polytetrafluorethylen (Deut.); politetrafluoretileno (Esp.); polyttrafluorthylne (Fr.); Teflon (Port.)
Examples: GORE-TEX [W.R.Gore]; Teflon TFE [DuPont]; Rulon [Saint-Gobain]; Algoflon [Solvay]; Fluorosint [Quadrant];
Applications
Personal Risks
Heated to temperatures consistent with everyday cooking, Teflon emits minute particles that can lodge deeply in the lungs. These particles are deadly to pet birds and can cause a disease known as polymer fume fever, a severe flu-like condition, in humans. Does not burn in flame but evaporates above 215C and evolves HF.
Environmental Risks
Physical and Chemical Properties
Highly resistant to acids, alkalis and solvents. Tenacity = 0.5-1.4 g/denier; Elongation = 15-32 %; Moisture regain = 0%; 1 mil films: 0.1 g/m2d
| Melting Point | 300 (dec) |
|---|---|
| Density | 2.1-2.3 |
Additional Information
DuPont: Teflon Website G.Cook, Handbook of Textile Fibres:II. Man-made Fibres, 5th edition, Merrow Publishing Co., Durham, England, 1984. p.509.
Sources Checked for Data in Record
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971
- Hoechst Celanese Corporation, Dictionary of Fiber & Textile Technology (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990
- Marjory L. Joseph, Introductory Textile Science, Holt, Rinehart and Winston, Fort Worth, TX, 1986
- Identification of Textile Materials, The Textile Institute, Manchester, England, 1985
- J.Gordon Cook, Handbook of Textile Fibres:II Man-made Fibres, Merrow Publishing Co. , Durham, England
- Theodore J. Reinhart, 'Glossary of Terms', Engineered Plastics, ASM International, 1988