Difference between revisions of "Terephthalic acid"
Jump to navigation
Jump to search
(username removed) |
|||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A white, crystalline compound. Terephthalic acid is used in the production of polyethylene terephthalate (polyester) [ | + | A white, crystalline compound. Terephthalic acid is used in the production of polyethylene terephthalate (polyester) [[polyester%20resin|resins]], [[polyester%20fiber|fibers]], and [[polyester%20film|films]]. |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| Line 9: | Line 9: | ||
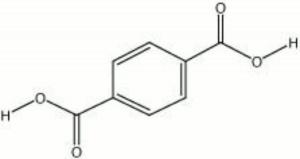
[[[SliderGallery rightalign|terephthalic acid.jpg~Chemical structure]]] | [[[SliderGallery rightalign|terephthalic acid.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| + | |||
| + | * Mild irritant. | ||
| + | * Combustible. Flash point = 260 C | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=AC180722500&productDescription=TEREPHTHALIC+ACID%2C+98%25+250GR&vendorId=VN00032119&countryCode=US&language=en SDS] | ||
| + | == Physical and Chemical Properties == | ||
Insoluble in water, chloroform, ether, acetic acid. Soluble in hot ethanol and alkalis. | Insoluble in water, chloroform, ether, acetic acid. Soluble in hot ethanol and alkalis. | ||
| Line 22: | Line 27: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 140.6 | + | | 140.6 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.51 | + | | 1.51 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 31: | Line 36: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 288 | + | | 288 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | + | * ''The Merck Index'', Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 9306 |
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 605 |
| − | * | + | * Hoechst Celanese Corporation, ''Dictionary of Fiber & Textile Technology'' (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 09:49, 8 June 2022
Description
A white, crystalline compound. Terephthalic acid is used in the production of polyethylene terephthalate (polyester) resins, fibers, and films.
Synonyms and Related Terms
1,4-benzenedicarboxylic acid; p-phthalic acid; Tephthol; TPA
Risks
- Mild irritant.
- Combustible. Flash point = 260 C
- ThermoFisher: SDS
Physical and Chemical Properties
Insoluble in water, chloroform, ether, acetic acid. Soluble in hot ethanol and alkalis.
| Composition | C6H4(COOH)2 |
|---|---|
| CAS | 100-21-0 |
| Melting Point | 140.6 C |
| Density | 1.51 g/ml |
| Molecular Weight | mol. wt.=166.1 |
| Boiling Point | 288 C |
Resources and Citations
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 9306
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 605
- Hoechst Celanese Corporation, Dictionary of Fiber & Textile Technology (older version called Man-made Fiber and Textile Dictionary, 1965), Hoechst Celanese Corporation, Charlotte NC, 1990
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993