Difference between revisions of "Tricresyl phosphate"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| Line 2: | Line 2: | ||
Colorless or pale yellow, oily liquid. Tricresyl phosphate is used as a solvent and plasticizer in [[polyvinyl%20chloride|polyvinyl chloride]], [[cellulose%20nitrate|cellulose nitrate]], [[mastic|mastic]] and some commercial lacquer formulations. It is also used industrially as a flame retardant, a [[lead|lead]] [[scavenger|scavenger]] in [[gasoline|gasoline]], and a waterproofing agent. | Colorless or pale yellow, oily liquid. Tricresyl phosphate is used as a solvent and plasticizer in [[polyvinyl%20chloride|polyvinyl chloride]], [[cellulose%20nitrate|cellulose nitrate]], [[mastic|mastic]] and some commercial lacquer formulations. It is also used industrially as a flame retardant, a [[lead|lead]] [[scavenger|scavenger]] in [[gasoline|gasoline]], and a waterproofing agent. | ||
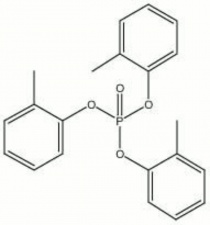
| − | + | [[[SliderGallery rightalign|tricresyl phosphate.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
TCP; tritolyl phosphate; phosphoric acid tris-(methylphenyl)ester; tri-o-cresyl phosphate; o-cresyl phosphate; o-tolyl phosphate; TOCP | TCP; tritolyl phosphate; phosphoric acid tris-(methylphenyl)ester; tri-o-cresyl phosphate; o-cresyl phosphate; o-tolyl phosphate; TOCP | ||
| − | + | == Risks == | |
| − | |||
| − | == | ||
| + | * Flame resistant but decomposes with heat to produce toxic fumes. | ||
| + | * Toxic by ingestion, inhalation and skin absorption. | ||
| + | * Flash point = 230C | ||
| + | * ThermoFisher: https://www.fishersci.com/store/msds?partNumber=T3421&productDescription=TRICRESYL+PHOSPHATE+TECH+1L&vendorId=VN00033897&countryCode=US&language=en SDS] | ||
| + | ==Physical and Chemical Properties== | ||
Miscible with organic solvents and oils. Insoluble in water. | Miscible with organic solvents and oils. Insoluble in water. | ||
| Line 22: | Line 25: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 11 | + | | 11 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 1.162 | + | | 1.162 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 34: | Line 37: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 275-280 | + | | 275-280 C |
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
* R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 | ||
Latest revision as of 13:06, 16 June 2022
Description
Colorless or pale yellow, oily liquid. Tricresyl phosphate is used as a solvent and plasticizer in Polyvinyl chloride, Cellulose nitrate, Mastic and some commercial lacquer formulations. It is also used industrially as a flame retardant, a Lead Scavenger in Gasoline, and a waterproofing agent.
Synonyms and Related Terms
TCP; tritolyl phosphate; phosphoric acid tris-(methylphenyl)ester; tri-o-cresyl phosphate; o-cresyl phosphate; o-tolyl phosphate; TOCP
Risks
- Flame resistant but decomposes with heat to produce toxic fumes.
- Toxic by ingestion, inhalation and skin absorption.
- Flash point = 230C
- ThermoFisher: https://www.fishersci.com/store/msds?partNumber=T3421&productDescription=TRICRESYL+PHOSPHATE+TECH+1L&vendorId=VN00033897&countryCode=US&language=en SDS]
Physical and Chemical Properties
Miscible with organic solvents and oils. Insoluble in water.
| Composition | (CH3C6H4O)3PO |
|---|---|
| CAS | 78-30-8 |
| Melting Point | 11 C |
| Density | 1.162 g/ml |
| Molecular Weight | mol. wt. =368.4 |
| Refractive Index | 1.556 |
| Boiling Point | 275-280 C |
Resources and Citations
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 135
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Hermann Kuhn, Conservation and Restoration of Works of Art and Antiquities, Butterworths, London, 1986
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9892