Difference between revisions of "Vermilion"
(Fixed internal links) |
|||
| Line 2: | Line 2: | ||
== Description == | == Description == | ||
| − | A bright red pigment composed of synthetically prepared [ | + | A bright red pigment composed of synthetically prepared [[Mercuric sulfide red| red mercuric sulfide]]. Vermilion is chemically identical to the mineral [[cinnabar]]. There are two methods, dry-process and wet-process, for preparing vermilion. The dry-process method was invented by the Chinese then imported to Europe about the 8th century seemingly via the Islamic world. Dry-process vermilion is prepared by adding [[ mercury]] to molten [[sulfur]], which forms a black isomorph of mercuric sulfide. The black mass is ground, heated to sublimation, then condensed to form vermilion. The wet-process method for making vermilion was developed in the late 17th century in Germany, but was not commercialized until the 19th century. The large scale production in the Netherlands in the 17th century was dry-process. Wet-process vermilion is made by placing the ground black mercuric sulfide isomorph into a solution of [[ammonium sulfide]] or [[potassium sulfide]]; this results in a red precipitate of vermilion. Vermilion is a very dense pigment with excellent hiding power and has been used in [[oil paint| oil]], [[watercolor paint|watercolor]], [[egg tempera]], and [[buon fresco|fresco]] paintings. Roman wall painting involved the prodigious use of cinnabar vermilion which came exclusively from famous mines in Spain (Almaden). It has been observed that when exposed to ultraviolet light and oxygen, vermilion can change from its normal red crystalline form to a gray or black product, but more recent research has indicated that the catalytic effect of chloride ions is probably more important than the role of light. The transformation which progresses via [[mercuric chloride]] species can result in dark, splotchy discolorations, common on egg tempera paintings (Gettens et al. 1993), but also in oil paint films, particularly when there is no surface glaze paint to protect the vermilion. By the 20th century, vermilion was superseded by [[cadmium red]] for most applications. |
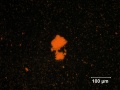
[[File:vermilion C100x.jpg|thumb|Vermilion]] | [[File:vermilion C100x.jpg|thumb|Vermilion]] | ||
Revision as of 19:09, 10 February 2016
Description
A bright red pigment composed of synthetically prepared red mercuric sulfide. Vermilion is chemically identical to the mineral Cinnabar. There are two methods, dry-process and wet-process, for preparing vermilion. The dry-process method was invented by the Chinese then imported to Europe about the 8th century seemingly via the Islamic world. Dry-process vermilion is prepared by adding Mercury to molten Sulfur, which forms a black isomorph of mercuric sulfide. The black mass is ground, heated to sublimation, then condensed to form vermilion. The wet-process method for making vermilion was developed in the late 17th century in Germany, but was not commercialized until the 19th century. The large scale production in the Netherlands in the 17th century was dry-process. Wet-process vermilion is made by placing the ground black mercuric sulfide isomorph into a solution of Ammonium sulfide or Potassium sulfide; this results in a red precipitate of vermilion. Vermilion is a very dense pigment with excellent hiding power and has been used in oil, watercolor, Egg tempera, and fresco paintings. Roman wall painting involved the prodigious use of cinnabar vermilion which came exclusively from famous mines in Spain (Almaden). It has been observed that when exposed to ultraviolet light and oxygen, vermilion can change from its normal red crystalline form to a gray or black product, but more recent research has indicated that the catalytic effect of chloride ions is probably more important than the role of light. The transformation which progresses via Mercuric chloride species can result in dark, splotchy discolorations, common on egg tempera paintings (Gettens et al. 1993), but also in oil paint films, particularly when there is no surface glaze paint to protect the vermilion. By the 20th century, vermilion was superseded by Cadmium red for most applications.
Synonyms and Related Terms
red mercuric sulfide; Pigment Red 106; CI 77766; cinnabar (mineral); Chinese vermilion (dry-process); English vermilion (wet-process); German vermilion (wet-process); Vermilion (Deut.); Zinnober (Deut.); vermillon (Fr.); vermellon (Esp.); bermellón (Esp.); kinnabaris (Gr.); cinabro (It.); vermiglio (It.); shu (Jap.); tan-sha (Chin.); dmar po (Tibetan); vermelhão (Port.); vermiljoen (Ned.); minium (obsolete usage); chusha; vermillion (sp); vermiculus;
Other Properties
Unaffected by most acids or alkalis. Soluble in aqua regia.
Hexagonal crystal system. Perfect cleavage in three directions (60 and 120 degree angles). Microscopically, vermillion made by the dry-proccess has irregular particles similar to ground cinnabar. Vermilion made by the wet-process has fine uniform particles that often form aggregates.
| Composition | HgS |
|---|---|
| Mohs Hardness | 2.0-2.5 |
| Density | 8.09-8.176 |
| Refractive Index | 2.854; 3.201 |
Hazards and Safety
Highly toxic by ingestion, inhalation and skin absorption. Darkens in ultraviolet light.
Additional Information
° R.Gettens, R.Feller, W.Chase, "Vermilion and Cinnabar", Artists Pigments, Volume 2, A. Roy (ed.), Oxford University Press: Oxford, 1993.° M.Spring and R.Grout, "The Blackening of Vermilion: An Analytical Study in the Process in Paintings", National Gallery Technical Bulletin Vol. 23, 2002, 50-61.Record content reviewed by EU-Artech, November 2007.
Additional Images
Authority
- External source or communication Comment: Submitted information: Ashok Roy, November 2007
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density = 8.09 and ref. index = 3.14; 2.81
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 611
- Reed Kay, The Painter's Guide To Studio Methods and Materials, Prentice-Hall, Inc., Englewood Cliffs, NJ, 1983
- Ralph Mayer, A Dictionary of Art Terms and Techniques, Harper and Row Publishers, New York, 1969 (also 1945 printing)
- R.D. Harley, Artists' Pigments c. 1600-1835, Butterworth Scientific, London, 1982
- Website address 1 Comment: Pigments Through the Ages - http://webexhibits.org/pigments/indiv/technical/vermilion.html - w = 2.905, e = 3.256; uniaxial
- Thomas B. Brill, Light Its Interaction with Art and Antiquities, Plenum Press, New York City, 1980 Comment: ref. index = 2.854, 3.201
- R.Feller, M.Curran, C.Bailie, 'Identification of Traditional Organic Colorants Employed in Japanese Prints and Determination of their Rates of Fading', Japanese Woodblock Prints, Allen Memorial Art Museum, Oberlin College, Oberlin, 1984 Comment: Japanese name
- The Dictionary of Art, Grove's Dictionaries Inc., New York, 1996 Comment: "Pigments"
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- Monona Rossol, The Artist's Complete Health and Safety Guide, Allworth Press, New York, 1994
- Book and Paper Group, Paper Conservation Catalog, AIC, 1984, 1989
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000