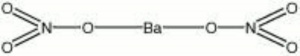
Barium nitrate
Jump to navigation
Jump to search
Description
White crystals or fused mass that is a strong oxidizing agent. . Barium nitrate is primarily used to produce green fireworks and green signal lights. It is also used in the manufacture of glass and ceramic glazes as well as for a rodenticide.
Risks
- Highly toxic by ingestion.
- Contact with skin and membranes may cause irritation.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in water. Insoluble in ethanol.
| Composition | Ba(NO3)2 |
|---|---|
| CAS | 10022-31-8 |
| Melting Point | 575 C |
| Density | 3.244 g/ml |
| Molecular Weight | mol. wt. = 261.34 |
Resources and Citations
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993 Comment: melting point=575C
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 1012
- Wikipedia: http://en.wikipedia.org/wiki/Barium_nitrate (Accessed Jan. 6, 2006) has melting point = 595C