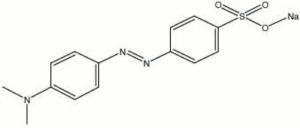
Methyl orange, sodium salt
Jump to navigation
Jump to search
Description
A pale, orange powder used as an acid-base
indicator. Methyl orange is an azo dye that was discovered by several workers near the same time period: P. Griess in 1875; O.N. Witt in 1876; and Z. Roussin in 1876. As an indicator, methyl orange forms a red color below pH 3.1, turns orange above pH 4.4 and becomes yellow in alkaline solutions. It is used for titrating
mineral acids. Methyl orange is also used for dyeing
textiles.
Synonyms and Related Terms
4-[[(4-dimethylamino)phenyl]-azo]benzenesulfonic acid sodium salt; sodium p-dimethylaminoazobenzenesulfonate; helianthine B; Acid Orange 52; CI 13025; Orange III; Gold Orange; Topaeolin D
Risks
- Toxic by ingestion.
- Inhalation and skin contact may cause irritation.
- ThermoFisher: SDS
Physical and Chemical Properties
Soluble in hot water. Insoluble in ethanol.
| Composition | (CH3)2NC6H4NNC6H4SO3Na |
|---|---|
| CAS | 547-58-0 |
| Density | 1.00 g/ml |
| Molecular Weight | mol. wt.=327.34 |
Resources and Citations
- The Merck Index, Susan Budavari (ed.), Merck Research Labs, Whitehouse Station, NJ, 12th Edition, 1996 Comment: entry 6180
- Colour Index International online at www.colour-index.org
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993