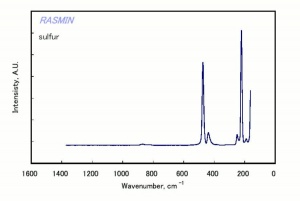
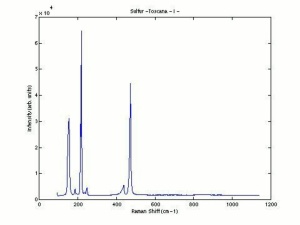
Sulfur
Description
A yellow nonmetallic element. Sulfur occurs naturally in several allotropes (primarily alpha and beta) as well as in many sulfate and sulfide minerals. Sulfur has an abundance of 0.05% in the earth's crust. It was known since ancient times. Some small Egyptian pieces appear to have been molded from molten sulfur. At one point sulfur was used as a gap-filler because it could be melted then poured into voids where it hardened into a plastic mass (Thornton 1998). Sulfur is available commercially in three high purity forms:
- Precipitated sulfur (milk of sulfur) - an amorphous powder formed by boiling sulfur with lime, then precipitating the solution with hydrochloric acid.
- Sublimed sulfur (flowers of sulfur) fine crystals formed by sublimation.
- Washed sulfur - fine crystals washed with ammonia to remove impurities.
Sulfur is used industrially for bleaching wood pulp, straw, wool, silk, felt, and linen. It is also used in vulcanizing rubber and making gunpowder. Sulfur is used in the manufacture of sulfa drugs, insecticides, plastic, enamels, and dyes.
Synonyms and Related Terms
S; sulphur (Br.); zwafvel (Ned.); soufre (Fr.); svovl (Dan.); Schwefel (Deut.); solfo (It.); enxofre (Port.); azufre (Esp.); svavel (Sven.); svovel (Nor.); brimstone; flower of sulfur; flour of sulfur; sublimed sulfur; milk of sulfur
Risks
- Combustible. Flash point = 207 C.
- Skin contact may cause irritation.
- Teck Resources: SDs
Physical and Chemical Properties
- Soluble in carbon disulfide, benzene, toluene, ammonium hydroxide. Slightly soluble in acetone, ethanol, ether. Insoluble in water.
- Burns with a blue flame and rotten egg smell.
- Orthorhombic system with tabular or steep bipyramidal crystals.
- Cleavage is poor.
- racture = conchioidal.
- Luster = greasy to adamantine.
- Streak = white
| Composition | S (atomic no. 16) |
|---|---|
| CAS | 7704-34-9 |
| Mohs Hardness | 1.5 - 2.5 |
| Melting Point | 112.8, 119.2 C |
| Density | 2.07, 1.96 g/ml |
| Molecular Weight | atomic wt = 32.065 |
| Boiling Point | 444.6 C |
Resources and Citations
- Sulfur Institute, 1140 Connecticut Ave. N.W., Washington DC 20006
- J.Thornton, "A Brief History and Review of the Early Practice and Materials of Gap-Filling in the West" JAIC 37: 3-22, 1998
- Mineralogy Database: Sulfur
- Web Elements: Website
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 783
- A.Lucas, J.R.Harris, Ancient Egyptian Materials and Industries, Edward Arnold Publishers Ltd., London, 4th edition, 1962
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 9142
- Encyclopedia Britannica, http://www.britannica.com Comment: "sulfur" [Accessed March 4, 2002]. -color photo
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- Wikipedia: http://en.wikipedia.org/wiki/Sulfur (Accessed Sept. 17, 2005)
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Dictionary of Building Preservation, Ward Bucher, ed., John Wiley & Sons, Inc., New York City, 1996
- The Dictionary of Paper, American Paper Institute, New York, Fourth Edition, 1980
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- AMOL reCollections Glossary -http://amol.org.au/recollections/7/c/htm