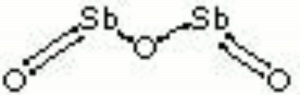Difference between revisions of "Antimony trioxide"
(username removed) |
|||
| (3 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
== Description == | == Description == | ||
| − | A white crystalline powder that occurs in nature as the mineral valentinite. Synthetic antimony trioxide, produced by roasting [ | + | A white crystalline powder that occurs in nature as the mineral valentinite. Synthetic antimony trioxide, produced by roasting [[antimony|antimony]] ore then mixing with [[barium%20sulfate|barium sulfate]] was introduced as an artists' pigment called antimony white and Timonox in 1919. It is inert, has good hiding power, and low oil absorption. Since it is darkened by [[hydrogen%20sulfide|hydrogen sulfide]], antimony oxide is often mixed with [[zinc%20oxide|zinc oxide]] which has preferential absorption for that gas (Gettens and Stout 1966). Some samples may contain senarmonite and/or valentinite, two known mineral forms of antimony oxide. Octahedral arsenic oxide may also be present as an impurity. Antimony trioxide is used as a white pigment and opacifiers in waxes, enamels, and glasses. It is also used to flameproof textiles, paper, and plastic. |
| − | + | [[[SliderGallery rightalign|antimony trioxide.jpg~Chemical structure]]] | |
== Synonyms and Related Terms == | == Synonyms and Related Terms == | ||
| − | antimony (III) oxide; antimony white; antimony oxide; Pigment White 11; Antimontrioxid (Deut.); Antimonweiss (Deut.); blanc d'antimoine (Fr.); oxyde d'antimoine (Fr.); leyko toy antimonioy (Gr.); bianco di antimonio (It.); triossido d'antimonio (It.); antimoon wit (Ned.); | + | antimony (III) oxide; antimony white; antimony oxide; Pigment White 11; Antimontrioxid (Deut.); Antimonweiss (Deut.); blanc d'antimoine (Fr.); oxyde d'antimoine (Fr.); leyko toy antimonioy (Gr.); bianco di antimonio (It.); triossido d'antimonio (It.); antimoon wit (Ned.); trióxido de antimónio (Port.); diantimony trioxide; antimony sesquioxide; flowers of antimony; exitelite; senarmontite; valentinite; weisspiessglanz; Timonox [Cookson Lead and Antimony, England]; |
| − | + | == Risks == | |
| − | == | + | * Highly toxic by inhalation and ingestion. |
| + | * Skin contact is corrosive. | ||
| + | * Fumes are carcinogenic. | ||
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=A860500&productDescription=ANTIMONY+TRIOXIDE+CERTIFD+500G&vendorId=VN00033897&countryCode=US&language=en SDS] | ||
| − | + | == Physical and Chemical Properties == | |
| − | Fine crystals (about 1 micron) appearing rounded or cubic. Colorless in plane-polarized light. Isotropic with low birefringence. | + | * Soluble in concentrated hydrochloric and sulfuric acids, strong alkalis. Insoluble in water. |
| + | * Fine crystals (about 1 micron) appearing rounded or cubic. | ||
| + | * Colorless in plane-polarized light. | ||
| + | * Isotropic with low birefringence. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 24: | Line 30: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 655 | + | | 655 |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 5.67-5.75 | + | | 5.67-5.75 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 36: | Line 42: | ||
|- | |- | ||
! scope="row"| Boiling Point | ! scope="row"| Boiling Point | ||
| − | | 1425 | + | | 1425 C |
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Comparisons == | == Comparisons == | ||
| Line 53: | Line 49: | ||
[[media:download_file_508.pdf|Characteristics of Common White Pigments]] | [[media:download_file_508.pdf|Characteristics of Common White Pigments]] | ||
| + | ==Resources and Citations== | ||
| + | * Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, ''Pigment Compendium'', Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: refractive index=2.087. Date for Timonox=1919 | ||
| − | + | * R. J. Gettens, G.L. Stout, ''Painting Materials, A Short Encyclopaedia'', Dover Publications, New York, 1966 Comment: density = 5.75 and ref. index = 2.18;2.35 | |
| − | |||
| − | * | ||
| − | |||
| − | |||
| − | * | + | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p. 64 |
| − | * | + | * Richard S. Lewis, ''Hawley's Condensed Chemical Dictionary'', Van Nostrand Reinhold, New York, 10th ed., 1993 |
| − | * | + | * Michael McCann, ''Artist Beware'', Watson-Guptill Publications, New York City, 1979 |
* ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 752 | * ''The Merck Index'', Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 752 | ||
Latest revision as of 13:31, 27 April 2022
Description
A white crystalline powder that occurs in nature as the mineral valentinite. Synthetic antimony trioxide, produced by roasting Antimony ore then mixing with Barium sulfate was introduced as an artists' pigment called antimony white and Timonox in 1919. It is inert, has good hiding power, and low oil absorption. Since it is darkened by Hydrogen sulfide, antimony oxide is often mixed with Zinc oxide which has preferential absorption for that gas (Gettens and Stout 1966). Some samples may contain senarmonite and/or valentinite, two known mineral forms of antimony oxide. Octahedral arsenic oxide may also be present as an impurity. Antimony trioxide is used as a white pigment and opacifiers in waxes, enamels, and glasses. It is also used to flameproof textiles, paper, and plastic.
Synonyms and Related Terms
antimony (III) oxide; antimony white; antimony oxide; Pigment White 11; Antimontrioxid (Deut.); Antimonweiss (Deut.); blanc d'antimoine (Fr.); oxyde d'antimoine (Fr.); leyko toy antimonioy (Gr.); bianco di antimonio (It.); triossido d'antimonio (It.); antimoon wit (Ned.); trióxido de antimónio (Port.); diantimony trioxide; antimony sesquioxide; flowers of antimony; exitelite; senarmontite; valentinite; weisspiessglanz; Timonox [Cookson Lead and Antimony, England];
Risks
- Highly toxic by inhalation and ingestion.
- Skin contact is corrosive.
- Fumes are carcinogenic.
- ThermoFisher: SDS
Physical and Chemical Properties
- Soluble in concentrated hydrochloric and sulfuric acids, strong alkalis. Insoluble in water.
- Fine crystals (about 1 micron) appearing rounded or cubic.
- Colorless in plane-polarized light.
- Isotropic with low birefringence.
| Composition | Sb2O3 |
|---|---|
| CAS | 1309-64-4 |
| Melting Point | 655 |
| Density | 5.67-5.75 g/ml |
| Molecular Weight | mol. wt. = 291.5 |
| Refractive Index | 2.18; 2.35 |
| Boiling Point | 1425 C |
Comparisons
Characteristics of Common White Pigments
Resources and Citations
- Nicholas Eastaugh, Valentine Walsh, Tracey Chaplin, Ruth Siddall, Pigment Compendium, Elsevier Butterworth-Heinemann, Oxford, 2004 Comment: refractive index=2.087. Date for Timonox=1919
- R. J. Gettens, G.L. Stout, Painting Materials, A Short Encyclopaedia, Dover Publications, New York, 1966 Comment: density = 5.75 and ref. index = 2.18;2.35
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p. 64
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- Michael McCann, Artist Beware, Watson-Guptill Publications, New York City, 1979
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983 Comment: entry 752
- Art and Architecture Thesaurus Online, http://www.getty.edu/research/tools/vocabulary/aat/, J. Paul Getty Trust, Los Angeles, 2000