Difference between revisions of "Sodium sulfate, anhydrous"
Jump to navigation
Jump to search
m (Text replace - "\[http:\/\/cameo\.mfa\.org\/materials\/fullrecord\.asp\?name=([^\s]+)\s(.*)\]" to "$2") |
|||
| Line 7: | Line 7: | ||
anhydrous sodium sulfate; anhydrous sodium sulphate (Br.); thenardite; salt cake | anhydrous sodium sulfate; anhydrous sodium sulphate (Br.); thenardite; salt cake | ||
| − | |||
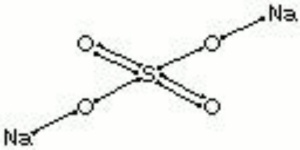
[[[SliderGallery rightalign|sodium sulfate, anhydrous.jpg~Chemical structure]]] | [[[SliderGallery rightalign|sodium sulfate, anhydrous.jpg~Chemical structure]]] | ||
| − | == | + | == Risks == |
| − | + | * Noncombustible. | |
| + | * ThermoFisher: [https://www.fishersci.com/store/msds?partNumber=S429500&productDescription=SOD+SULFATE+ANHYD+USP%2FFCC+500G&vendorId=VN00033897&countryCode=US&language=en SDS] | ||
| − | Orthorhombic crystals with perfect cleavage in one direction. | + | == Physical and Chemical Properties == |
| − | + | * Soluble in water, glycerol. Insoluble in ethanol. | |
| − | Mineral thenardite is vitreous, translucent and produces a white streak. | + | * Orthorhombic crystals with perfect cleavage in one direction. |
| + | * Mineral thenardite is vitreous, translucent and produces a white streak. | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 30: | Line 31: | ||
|- | |- | ||
! scope="row"| Melting Point | ! scope="row"| Melting Point | ||
| − | | 888 | + | | 888 C |
|- | |- | ||
! scope="row"| Density | ! scope="row"| Density | ||
| − | | 2.671 | + | | 2.671 g/ml |
|- | |- | ||
! scope="row"| Molecular Weight | ! scope="row"| Molecular Weight | ||
| Line 39: | Line 40: | ||
|} | |} | ||
| − | == | + | ==Resources and Citations== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * Grossi, "Acoustic emission monitoring to study sodium sulfate crystallization in monumental porous carbonate stone" ''Studies in Conservation'' 42 (1997), p. 115-125. | |
* G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p.785 | * G.S.Brady, ''Materials Handbook'', McGraw-Hill Book Co., New York, 1971 Comment: p.785 | ||
| Line 65: | Line 58: | ||
* ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | * ''The American Heritage Dictionary'' or ''Encarta'', via Microsoft Bookshelf 98, Microsoft Corp., 1998 | ||
| − | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "thenardite." | + | * ''Encyclopedia Britannica'', http://www.britannica.com Comment: "thenardite." Accessed 7 Apr. 2005 . |
* C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | * C.W.Chesterman, K.E.Lowe, ''Audubon Society Field Guide to North American Rocks and Minerals'', Alfred A. Knopf, New York, 1979 | ||
| Line 73: | Line 66: | ||
* Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | * Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, ''Technology and Conservation'', Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985 | ||
| − | * | + | * Photographic chemicals at www.jetcity.com/~mrjones/chemdesc.htm |
[[Category:Materials database]] | [[Category:Materials database]] | ||
Latest revision as of 10:22, 2 June 2022
Description
White, hygroscopic crystals or powder. Anhydrous sodium sulfate occurs in nature as the mineral thenardite. Thenardite occurs most often as an evaporation product near salt lakes and playas, and is mined in arid regions of northern Africa, Siberia, Canada, and the western United States. Anhydrous sodium sulfate is used in the manufacture of Kraft paper, Paperboard, Glass, synthetic ultramarine blue, and ceramic Glaze. It is also used as a leveling agent in dyeing textiles to ensure even color acceptance.
Synonyms and Related Terms
anhydrous sodium sulfate; anhydrous sodium sulphate (Br.); thenardite; salt cake
Risks
- Noncombustible.
- ThermoFisher: SDS
Physical and Chemical Properties
- Soluble in water, glycerol. Insoluble in ethanol.
- Orthorhombic crystals with perfect cleavage in one direction.
- Mineral thenardite is vitreous, translucent and produces a white streak.
| Composition | Na2SO4 |
|---|---|
| CAS | 7757-82-6 |
| Mohs Hardness | 2.5-3.0 |
| Melting Point | 888 C |
| Density | 2.671 g/ml |
| Molecular Weight | mol. wt. = 142.1 |
Resources and Citations
- Grossi, "Acoustic emission monitoring to study sodium sulfate crystallization in monumental porous carbonate stone" Studies in Conservation 42 (1997), p. 115-125.
- G.S.Brady, Materials Handbook, McGraw-Hill Book Co., New York, 1971 Comment: p.785
- Richard S. Lewis, Hawley's Condensed Chemical Dictionary, Van Nostrand Reinhold, New York, 10th ed., 1993
- The Dictionary of Paper, American Paper Institute, New York, Fourth Edition, 1980
- Van Nostrand's Scientific Encyclopedia, Douglas M. Considine (ed.), Van Nostrand Reinhold, New York, 1976
- Random House, Webster's Encyclopedic Unabridged Dictionary of the English Language, Grammercy Book, New York, 1997
- The Merck Index, Martha Windholz (ed.), Merck Research Labs, Rahway NJ, 10th edition, 1983
- The American Heritage Dictionary or Encarta, via Microsoft Bookshelf 98, Microsoft Corp., 1998
- Encyclopedia Britannica, http://www.britannica.com Comment: "thenardite." Accessed 7 Apr. 2005 .
- C.W.Chesterman, K.E.Lowe, Audubon Society Field Guide to North American Rocks and Minerals, Alfred A. Knopf, New York, 1979
- John and Margaret Cannon, Dye Plants and Dyeing, Herbert Press, London, 1994
- Susan E. Schur, Conservation Terminology: A review of Past & Current Nomenclature of Materials, Technology and Conservation, Spring (p.34-39); Summer (p.35-38); Fall (p.25-36), 1985
- Photographic chemicals at www.jetcity.com/~mrjones/chemdesc.htm